NPAS1 represses the generation of specific subtypes of cortical interneurons
- PMID: 25467980
- PMCID: PMC4258152
- DOI: 10.1016/j.neuron.2014.10.040
NPAS1 represses the generation of specific subtypes of cortical interneurons
Abstract
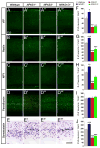
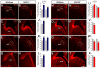
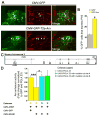
Little is known about genetic mechanisms that regulate the ratio of cortical excitatory and inhibitory neurons. We show that NPAS1 and NPAS3 transcription factors (TFs) are expressed in progenitor domains of the mouse basal ganglia (subpallium, MGE, and CGE). NPAS1(-/-) mutants had increased proliferation, ERK signaling, and expression of Arx in the MGE and CGE. NPAS1(-/-) mutants also had increased neocortical inhibition (sIPSC and mIPSC) and generated an excess of somatostatin(+) (SST) (MGE-derived) and vasoactive intestinal polypeptide(+) (VIP) (CGE-derived) neocortical interneurons, but had a normal density of parvalbumin(+) (PV) (MGE-derived) interneurons. In contrast, NPAS3(-/-) mutants showed decreased proliferation and ERK signaling in progenitors of the ganglionic eminences and had fewer SST(+) and VIP(+) interneurons. NPAS1 repressed activity of an Arx enhancer, and Arx overexpression resulted in increased proliferation of CGE progenitors. These results provide insights into genetic regulation of cortical interneuron numbers and cortical inhibitory tone.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Acsády L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996;73:299–315. - PubMed
-
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 NS085046/NS/NINDS NIH HHS/United States
- R01 NS071785/NS/NINDS NIH HHS/United States
- T32 MH089920/MH/NIMH NIH HHS/United States
- F32 NS077747/NS/NINDS NIH HHS/United States
- R01EB009756/EB/NIBIB NIH HHS/United States
- R37 MH049428/MH/NIMH NIH HHS/United States
- F32NS077747/NS/NINDS NIH HHS/United States
- R01 MH081880/MH/NIMH NIH HHS/United States
- R01NS071785/NS/NINDS NIH HHS/United States
- R01 EB009756/EB/NIBIB NIH HHS/United States
- 5-R01-MH087986/MH/NIMH NIH HHS/United States
- R01 MH087986/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

