3D traction stresses activate protease-dependent invasion of cancer cells
- PMID: 25468332
- PMCID: PMC4255420
- DOI: 10.1016/j.bpj.2014.07.078
3D traction stresses activate protease-dependent invasion of cancer cells
Abstract
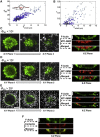
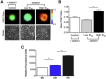
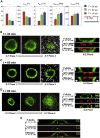
Cell invasion and migration that occurs, for example, in cancer metastasis is rooted in the ability of cells to navigate through varying levels of physical constraint exerted by the extracellular matrix. Cancer cells can invade matrices in either a protease-independent or a protease-dependent manner. An emerging critical component that influences the mode of cell invasion is the traction stresses generated by the cells in response to the physicostructural properties of the extracellular matrix. In this study, we have developed a reference-free quantitative assay for measuring three-dimensional (3D) traction stresses generated by cells during the initial stages of invasion into matrices exerting varying levels of mechanical resistance. Our results show that as cells encounter higher mechanical resistance, a larger fraction of them shift to protease-mediated invasion, and this process begins at lower values of cell invasion depth. On the other hand, the compressive stress generated by the cells at the onset of protease-mediated invasion is found to be independent of matrix stiffness, suggesting that 3D traction stress is a key factor in triggering protease-mediated cancer cell invasion. At low 3D compressive traction stresses, cells utilize bleb formation to indent the matrix in a protease independent manner. However, at higher stress values, cells utilize invadopodia-like structures to mediate protease-dependent invasion into the 3D matrix. The critical value of compressive traction stress at the transition from a protease-independent to a protease-dependent mode of invasion is found to be ∼165 Pa.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Switching from protease-independent to protease-dependent cancer cell invasion.Biophys J. 2014 Dec 2;107(11):2484-5. doi: 10.1016/j.bpj.2014.10.047. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468324 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

