Fiber-dependent and -independent toxicity of islet amyloid polypeptide
- PMID: 25468335
- PMCID: PMC4255681
- DOI: 10.1016/j.bpj.2014.09.047
Fiber-dependent and -independent toxicity of islet amyloid polypeptide
Abstract
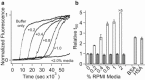
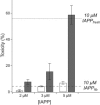
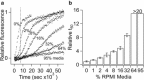
The 37-residue peptide hormone islet amyloid polypeptide (IAPP) plays a central role in diabetes pathology. Although its amyloid fiber aggregation kinetics and cytotoxicity to β-cells are well documented, few reports have directly assessed the role of fibers in cell-based toxicity experiments. Here, we report that amyloid formation of IAPP can be strongly inhibited by the extracellular environment of live cells. For example, fiber formation is more strongly suppressed in cell culture medium than in aqueous buffer. The serum component of the medium is responsible for this inhibition. Although amyloid formation was previously shown to be catalyzed by both synthetic and chloroform-extracted phospholipid surfaces, it is instead inhibited by membrane surfaces prepared directly from the plasma membranes of an immortal β-cell line. This disparity is reconciled by direct assessment of fibers in cell-culture-based toxicity experiments. We discovered that fibers are nontoxic if they are washed free of adsorbed nonfibrillar components. Moreover, toxicity is not only rescued when monomers are added back to fibers but is greater than what is observed from the precursor alone. Our results are interpreted in light of the capacity of the fiber surface to template amyloid nucleation.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Höppener J.W.M., Ahrén B., Lips C.J.M. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2000;343:411–419. - PubMed
-
- Kahn S.E., Andrikopoulos S., Verchere C.B. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. - PubMed
-
- Matveyenko A.V., Butler P.C. β-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106–2114. - PubMed
-
- Farese R.V., DiMarco P.E., Morrison A.D. Rapid glucose-dependent increases in phosphatidic acid and phosphoinositides in rat pancreatic islets. Endocrinology. 1986;118:1498–1503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

