Loss of function in heparan sulfate elongation genes EXT1 and EXT 2 results in improved nitric oxide bioavailability and endothelial function
- PMID: 25468659
- PMCID: PMC4338717
- DOI: 10.1161/JAHA.114.001274
Loss of function in heparan sulfate elongation genes EXT1 and EXT 2 results in improved nitric oxide bioavailability and endothelial function
Abstract
Background: Heparanase is the major enzyme involved in degradation of endothelial heparan sulfates, which is associated with impaired endothelial nitric oxide synthesis. However, the effect of heparan sulfate chain length in relation to endothelial function and nitric oxide availability has never been investigated. We studied the effect of heterozygous mutations in heparan sulfate elongation genes EXT1 and EXT2 on endothelial function in vitro as well as in vivo.
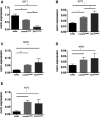
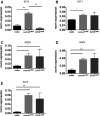
Methods and result: Flow-mediated dilation, a marker of nitric oxide bioavailability, was studied in Ext1(+/-) and Ext2(+/-) mice versus controls (n=7 per group), as well as in human subjects with heterozygous loss of function mutations in EXT1 and EXT2 (n=13 hereditary multiple exostoses and n=13 controls). Endothelial function was measured in microvascular endothelial cells under laminar flow with or without siRNA targeting EXT1 or EXT2. Endothelial glycocalyx and maximal arteriolar dilatation were significantly altered in Ext1(+/-) and Ext2(+/-) mice compared to wild-type littermates (glycocalyx: wild-type 0.67±0.1 μm, Ext1(+/-) 0.28±0.1 μm and Ext2(+/-) 0.25±0.1 μm, P<0.01, maximal arteriolar dilation during reperfusion: wild-type 11.3±1.0%), Ext1(+/-) 15.2±1.4% and Ext2(+/-) 13.8±1.6% P<0.05). In humans, brachial artery flow-mediated dilation was significantly increased in hereditary multiple exostoses patients (hereditary multiple exostoses 8.1±0.8% versus control 5.6±0.7%, P<0.05). In line, silencing of microvascular endothelial cell EXT1 and EXT2 under flow led to significant upregulation of endothelial nitric oxide synthesis and phospho-endothelial nitric oxide synthesis protein expression.
Conclusions: Our data implicate that heparan sulfate elongation genes EXT1 and EXT2 are involved in maintaining endothelial homeostasis, presumably via increased nitric oxide bioavailability.
Keywords: EXT; endothelial function; heparan sulfate; nitric oxide.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures



References
-
- Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, Bennett D, Bernabe E, Blencowe H, Bourne R, Braithwaite T, Brayne C, Bruce NG, Brugha TS, Burney P, Dherani M, Dolk H, Edmond K, Ezzati M, Flaxman AD, Fleming TD, Freedman G, Gunnell D, Hay RJ, Hutchings SJ, Ohno SL, Lozano R, Lyons RA, Marcenes W, Naghavi M, Newton CR, Pearce N, Pope D, Rushton L, Salomon JA, Shibuya K, Vos T, Wang H, Williams HC, Woolf AD, Lopez AD, Davis A. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet. 2013; 381:997-1020. - PubMed
-
- Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano‐sensor and ‐transducer. Sci Signal. 2008; 1:pt8. - PubMed
-
- Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear‐induced endothelium‐derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003; 285:H722-H726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

