A Food and Drug Administration-approved asthma therapeutic agent impacts amyloid β in the brain in a transgenic model of Alzheimer disease
- PMID: 25468905
- PMCID: PMC4303653
- DOI: 10.1074/jbc.M114.586602
A Food and Drug Administration-approved asthma therapeutic agent impacts amyloid β in the brain in a transgenic model of Alzheimer disease
Abstract
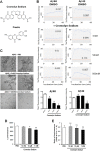
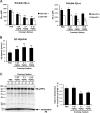
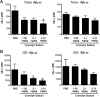
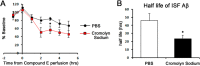
Interfering with the assembly of Amyloid β (Aβ) peptides from monomer to oligomeric species and fibrils or promoting their clearance from the brain are targets of anti-Aβ-directed therapies in Alzheimer disease. Here we demonstrate that cromolyn sodium (disodium cromoglycate), a Food and Drug Administration-approved drug already in use for the treatment of asthma, efficiently inhibits the aggregation of Aβ monomers into higher-order oligomers and fibrils in vitro without affecting Aβ production. In vivo, the levels of soluble Aβ are decreased by over 50% after only 1 week of daily intraperitoneally administered cromolyn sodium. Additional in vivo microdialysis studies also show that this compound decreases the half-life of soluble Aβ in the brain. These data suggest a clear effect of a peripherally administered, Food and Drug Administration-approved medication on Aβ economy, supporting further investigation of the potential long-term efficacy of cromolyn sodium in Alzheimer disease.
Keywords: Aggregation; Alzheimer Disease; Amyloid β (AB); Cromolyn Sodium; asthma; in vivo microdialysis; mouse.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Cromolyn Reduces Levels of the Alzheimer's Disease-Associated Amyloid β-Protein by Promoting Microglial Phagocytosis.Sci Rep. 2018 Jan 18;8(1):1144. doi: 10.1038/s41598-018-19641-2. Sci Rep. 2018. PMID: 29348604 Free PMC article.
-
High order W02-reactive stable oligomers of amyloid-β are produced in vivo and in vitro via dialysis and filtration of synthetic amyloid-β monomer.J Alzheimers Dis. 2015;44(1):69-78. doi: 10.3233/JAD-132024. J Alzheimers Dis. 2015. PMID: 25182735
-
Evaluation of Fluorinated Cromolyn Derivatives as Potential Therapeutics for Alzheimer's Disease.J Alzheimers Dis. 2021;80(2):775-786. doi: 10.3233/JAD-201419. J Alzheimers Dis. 2021. PMID: 33579853
-
Low-n oligomers as therapeutic targets of Alzheimer's disease.J Neurochem. 2011 Apr;117(1):19-28. doi: 10.1111/j.1471-4159.2011.07187.x. Epub 2011 Feb 9. J Neurochem. 2011. PMID: 21244429 Review.
-
Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease.Neurol Res. 2005 Dec;27(8):869-81. doi: 10.1179/016164105X49436. Neurol Res. 2005. PMID: 16354549 Review.
Cited by
-
Review of Advanced Drug Trials Focusing on the Reduction of Brain Beta-Amyloid to Prevent and Treat Dementia.J Exp Pharmacol. 2022 Oct 30;14:331-352. doi: 10.2147/JEP.S265626. eCollection 2022. J Exp Pharmacol. 2022. PMID: 36339394 Free PMC article. Review.
-
Cromolyn prevents cerebral vasospasm and dementia by targeting WDR43.Front Aging Neurosci. 2023 Apr 13;15:1132733. doi: 10.3389/fnagi.2023.1132733. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37122373 Free PMC article.
-
Marine Natural Products, Multitarget Therapy and Repurposed Agents in Alzheimer's Disease.Pharmaceuticals (Basel). 2020 Sep 11;13(9):242. doi: 10.3390/ph13090242. Pharmaceuticals (Basel). 2020. PMID: 32933034 Free PMC article. Review.
-
Cromolyn sodium delays disease onset and is neuroprotective in the SOD1G93A Mouse Model of amyotrophic lateral sclerosis.Sci Rep. 2019 Nov 27;9(1):17728. doi: 10.1038/s41598-019-53982-w. Sci Rep. 2019. PMID: 31776380 Free PMC article.
-
The Evaluation of Concentration - In-Vitro Release Relationship for Topical Semisolid Formulations of Sodium Cromoglycate.Curr Health Sci J. 2015 Oct-Dec;41(4):368-374. doi: 10.12865/CHSJ.41.04.12. Epub 2015 Dec 22. Curr Health Sci J. 2015. PMID: 30538844 Free PMC article.
References
-
- Anand P., Singh B. (2013) A review on cholinesterase inhibitors for Alzheimer's disease. Arch. Pharm. Res. 36, 375–399 - PubMed
-
- Doody R. S., Ferris S. H., Salloway S., Sun Y., Goldman R., Watkins W. E., Xu Y., Murthy A. K. (2009) Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology 72, 1555–1561 - PubMed
-
- Cummings J. L. (2004) Treatment of Alzheimer's disease: current and future therapeutic approaches. Rev. Neurol. Dis. 1, 60–69 - PubMed
-
- Selkoe D. J. (2001) Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimers Dis. 3, 75–80 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

