Characterization of Cav1.4 complexes (α11.4, β2, and α2δ4) in HEK293T cells and in the retina
- PMID: 25468907
- PMCID: PMC4340398
- DOI: 10.1074/jbc.M114.607465
Characterization of Cav1.4 complexes (α11.4, β2, and α2δ4) in HEK293T cells and in the retina
Abstract
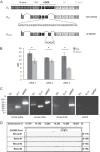
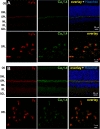
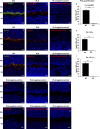
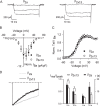
In photoreceptor synaptic terminals, voltage-gated Cav1.4 channels mediate Ca(2+) signals required for transmission of visual stimuli. Like other high voltage-activated Cav channels, Cav1.4 channels are composed of a main pore-forming Cav1.4 α1 subunit and auxiliary β and α2δ subunits. Of the four distinct classes of β and α2δ, β2 and α2δ4 are thought to co-assemble with Cav1.4 α1 subunits in photoreceptors. However, an understanding of the functional properties of this combination of Cav subunits is lacking. Here, we provide evidence that Cav1.4 α1, β2, and α2δ4 contribute to Cav1.4 channel complexes in the retina and describe their properties in electrophysiological recordings. In addition, we identified a variant of β2, named here β2X13, which, along with β2a, is present in photoreceptor terminals. Cav1.4 α1, β2, and α2δ4 were coimmunoprecipitated from lysates of transfected HEK293 cells and mouse retina and were found to interact in the outer plexiform layer of the retina containing the photoreceptor synaptic terminals, by proximity ligation assays. In whole-cell patch clamp recordings of transfected HEK293T cells, channels (Cav1.4 α1 + β2X13) containing α2δ4 exhibited weaker voltage-dependent activation than those with α2δ1. Moreover, compared with channels (Cav1.4 α1 + α2δ4) with β2a, β2X13-containing channels exhibited greater voltage-dependent inactivation. The latter effect was specific to Cav1.4 because it was not seen for Cav1.2 channels. Our results provide the first detailed functional analysis of the Cav1.4 subunits that form native photoreceptor Cav1.4 channels and indicate potential heterogeneity in these channels conferred by β2a and β2X13 variants.
Keywords: Calcium Channel; Calcium-binding Protein; Electrophysiology; Immunohistochemistry; Photoreceptor; Retina.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Functional impact of a congenital stationary night blindness type 2 mutation depends on subunit composition of Cav1.4 Ca2+ channels.J Biol Chem. 2020 Dec 11;295(50):17215-17226. doi: 10.1074/jbc.RA120.014138. Epub 2020 Oct 8. J Biol Chem. 2020. PMID: 33037074 Free PMC article.
-
Genetic, cellular, and functional evidence for Ca2+ inflow through Cav1.2 and Cav1.3 channels in murine spiral ganglion neurons.J Neurosci. 2014 May 21;34(21):7383-93. doi: 10.1523/JNEUROSCI.5416-13.2014. J Neurosci. 2014. PMID: 24849370 Free PMC article.
-
Pediatric Dilated Cardiomyopathy-Associated LRRC10 (Leucine-Rich Repeat-Containing 10) Variant Reveals LRRC10 as an Auxiliary Subunit of Cardiac L-Type Ca2+ Channels.J Am Heart Assoc. 2018 Feb 3;7(3):e006428. doi: 10.1161/JAHA.117.006428. J Am Heart Assoc. 2018. PMID: 29431102 Free PMC article.
-
Interventions for central serous chorioretinopathy: a network meta-analysis.Cochrane Database Syst Rev. 2025 Jun 16;6(6):CD011841. doi: 10.1002/14651858.CD011841.pub3. Cochrane Database Syst Rev. 2025. PMID: 40522203
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Retinoschisin Facilitates the Function of L-Type Voltage-Gated Calcium Channels.Front Cell Neurosci. 2017 Aug 8;11:232. doi: 10.3389/fncel.2017.00232. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28848397 Free PMC article.
-
Pathophysiological Roles of Auxiliary Calcium Channel α2δ Subunits.Handb Exp Pharmacol. 2023;279:289-316. doi: 10.1007/164_2022_630. Handb Exp Pharmacol. 2023. PMID: 36598609
-
A dual role for Cav1.4 Ca2+ channels in the molecular and structural organization of the rod photoreceptor synapse.Elife. 2020 Sep 17;9:e62184. doi: 10.7554/eLife.62184. Elife. 2020. PMID: 32940604 Free PMC article.
-
The Contribution of L-Type Cav1.3 Channels to Retinal Light Responses.Front Mol Neurosci. 2017 Dec 5;10:394. doi: 10.3389/fnmol.2017.00394. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29259539 Free PMC article.
-
Voltage-Gated Calcium Channels: Key Players in Sensory Coding in the Retina and the Inner Ear.Physiol Rev. 2018 Oct 1;98(4):2063-2096. doi: 10.1152/physrev.00030.2017. Physiol Rev. 2018. PMID: 30067155 Free PMC article. Review.
References
-
- Dolphin A. C. (2012) Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat. Rev. Neurosci. 13, 542–555 - PubMed
-
- Dolphin A. C. (2013) The α2δ subunits of voltage-gated calcium channels. Biochim. Biophys. Acta 1828, 1541–1549 - PubMed
-
- Simms B. A., Zamponi G. W. (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82, 24–45 - PubMed
-
- Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

