Glycogen synthase kinase 3β orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion
- PMID: 25468908
- PMCID: PMC4340382
- DOI: 10.1074/jbc.M114.593830
Glycogen synthase kinase 3β orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion
Abstract
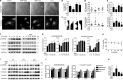
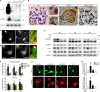
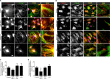
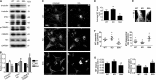
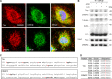
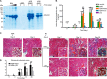
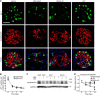
Reminiscent of neural repair, following podocyte depletion, remnant-surviving podocytes exhibit a considerable adaptive capacity to expand and cover the denuded renal glomerular basement membrane. Microtubules, one of the principal cytoskeletal components of podocyte major processes, play a crucial role in podocyte morphogenesis and podocyte process outgrowth, branching, and elongation. Here, we demonstrated that the microtubule-associated proteins Tau and collapsin response mediator protein (CRMP) 2, key regulators of microtubule dynamics, were abundantly expressed by glomerular podocytes in vivo and in vitro, interacted with glycogen synthase kinase (GSK)3β, and served as its putative substrates. GSK3β overactivity induced by adriamycin injury or by a constitutively active mutant of GSK3β augmented phosphorylation of Tau and CRMP2, concomitant with microtubule depolymerization, cell body shrinkage, and shortening of podocyte processes. Conversely, inhibition of GSK3β by a dominant negative mutant or by lithium, a Food and Drug Administration-approved neuroprotective mood stabilizer, diminished Tau and CRMP2 phosphorylation, resulting in microtubule polymerization, podocyte expansion, and lengthening of podocyte processes. In a mouse model of adriamycin-induced podocyte depletion and nephropathy, delayed administration of a single low dose of lithium attenuated proteinuria and ameliorated progressive glomerulosclerosis despite no correction of podocytopenia. Mechanistically, lithium therapy obliterated GSK3β overactivity, mitigated phosphorylation of Tau and CRMP2, and enhanced microtubule polymerization and stabilization in glomeruli in adriamycin-injured kidneys, associated with elongation of podocyte major processes. Collectively, our findings suggest that the GSK3β-dictated podocyte microtubule dynamics might serve as a novel therapeutic target to reinforce the compensatory glomerular adaptation to podocyte loss.
Keywords: Cytoskeleton; Glycogen Synthase Kinase 3β; Microtubule; Microtubule-associated Protein (MAP); Podocyte; Proteinuria; Tau Protein (Tau).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Kriz W. (2012) Glomerular diseases: podocyte hypertrophy mismatch and glomerular disease. Nat. Rev. Nephrol. 8, 618–619 - PubMed
-
- D'Agati V. D., Kaskel F. J., Falk R. J. (2011) Focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 2398–2411 - PubMed
-
- D'Agati V. D. (2012) Pathobiology of focal segmental glomerulosclerosis: new developments. Curr. Opin. Nephrol. Hypertens. 21, 243–250 - PubMed
-
- Wharram B. L., Goyal M., Wiggins J. E., Sanden S. K., Hussain S., Filipiak W. E., Saunders T. L., Dysko R. C., Kohno K., Holzman L. B., Wiggins R. C. (2005) Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941–2952 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

