Impact of retinoic acid exposure on midfacial shape variation and manifestation of holoprosencephaly in Twsg1 mutant mice
- PMID: 25468951
- PMCID: PMC4314779
- DOI: 10.1242/dmm.018275
Impact of retinoic acid exposure on midfacial shape variation and manifestation of holoprosencephaly in Twsg1 mutant mice
Abstract
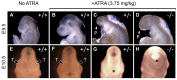
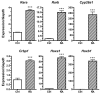
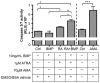
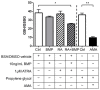
Holoprosencephaly (HPE) is a developmental anomaly characterized by inadequate or absent midline division of the embryonic forebrain and midline facial defects. It is believed that interactions between genes and the environment play a role in the widely variable penetrance and expressivity of HPE, although direct investigation of such effects has been limited. The goal of this study was to examine whether mice carrying a mutation in a gene encoding the bone morphogenetic protein (BMP) antagonist twisted gastrulation (Twsg1), which is associated with a low penetrance of HPE, are sensitized to retinoic acid (RA) teratogenesis. Pregnant Twsg1(+/-) dams were treated by gavage with a low dose of all-trans RA (3.75 mg/kg of body weight). Embryos were analyzed between embryonic day (E)9.5 and E11.5 by microscopy and geometric morphometric analysis by micro-computed tomography. P19 embryonal carcinoma cells were used to examine potential mechanisms mediating the combined effects of increased BMP and retinoid signaling. Although only 7% of wild-type embryos exposed to RA showed overt HPE or neural tube defects (NTDs), 100% of Twsg1(-/-) mutants exposed to RA manifested severe HPE compared to 17% without RA. Remarkably, up to 30% of Twsg1(+/-) mutants also showed HPE (23%) or NTDs (7%). The majority of shape variation among Twsg1(+/-) mutants was associated with narrowing of the midface. In P19 cells, RA induced the expression of Bmp2, acted in concert with BMP2 to increase p53 expression, caspase activation and oxidative stress. This study provides direct evidence for modifying effects of the environment in a genetic mouse model carrying a predisposing mutation for HPE in the Twsg1 gene. Further study of the mechanisms underlying these gene-environment interactions in vivo will contribute to better understanding of the pathogenesis of birth defects and present an opportunity to explore potential preventive interventions.
Keywords: Apoptosis; Bone morphogenetic protein; Holoprosencephaly; Oxidative stress; Retinoic acid; Twisted gastrulation; Twsg1.
© 2015. Published by The Company of Biologists Ltd.
Figures


References
-
- Anderson R. M., Lawrence A. R., Stottmann R. W., Bachiller D., Klingensmith J. (2002). Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 129, 4975–4987. - PubMed
-
- Andersson O., Reissmann E., Jörnvall H., Ibáñez C. F. (2006). Synergistic interaction between Gdf1 and Nodal during anterior axis development. Dev. Biol. 293, 370–381. - PubMed
-
- Aoto K., Shikata Y., Higashiyama D., Shiota K., Motoyama J. (2008). Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res. A Clin. Mol. Teratol. 82, 224–231. - PubMed
-
- Balmer J. E., Blomhoff R. (2002). Gene expression regulation by retinoic acid. J. Lipid Res. 43, 1773–1808. - PubMed
-
- Billington C. J., Jr, Ng B., Forsman C., Schmidt B., Bagchi A., Symer D. E., Schotta G., Gopalakrishnan R., Sarver A. L., Petryk A. (2011). The molecular and cellular basis of variable craniofacial phenotypes and their genetic rescue in Twisted gastrulation mutant mice. Dev. Biol. 355, 21–31. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

