Type IV pili interactions promote intercellular association and moderate swarming of Pseudomonas aeruginosa
- PMID: 25468980
- PMCID: PMC4273417
- DOI: 10.1073/pnas.1414661111
Type IV pili interactions promote intercellular association and moderate swarming of Pseudomonas aeruginosa
Abstract
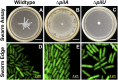
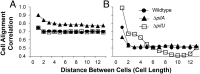
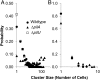
Pseudomonas aeruginosa is a ubiquitous bacterium that survives in many environments, including as an acute and chronic pathogen in humans. Substantial evidence shows that P. aeruginosa behavior is affected by its motility, and appendages known as flagella and type IV pili (TFP) are known to confer such motility. The role these appendages play when not facilitating motility or attachment, however, is unclear. Here we discern a passive intercellular role of TFP during flagellar-mediated swarming of P. aeruginosa that does not require TFP extension or retraction. We studied swarming at the cellular level using a combination of laboratory experiments and computational simulations to explain the resultant patterns of cells imaged from in vitro swarms. Namely, we used a computational model to simulate swarming and to probe for individual cell behavior that cannot currently be otherwise measured. Our simulations showed that TFP of swarming P. aeruginosa should be distributed all over the cell and that TFP-TFP interactions between cells should be a dominant mechanism that promotes cell-cell interaction, limits lone cell movement, and slows swarm expansion. This predicted physical mechanism involving TFP was confirmed in vitro using pairwise mixtures of strains with and without TFP where cells without TFP separate from cells with TFP. While TFP slow swarm expansion, we show in vitro that TFP help alter collective motion to avoid toxic compounds such as the antibiotic carbenicillin. Thus, TFP physically affect P. aeruginosa swarming by actively promoting cell-cell association and directional collective motion within motile groups to aid their survival.
Keywords: biofilms; collective motion; computational model; predictive simulations; self-organization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gaynes R, Edwards JR. National Nosocomial Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

