Boceprevir is highly effective in treatment-experienced hepatitis C virus-positive genotype-1 menopausal women
- PMID: 25469044
- PMCID: PMC4248219
- DOI: 10.3748/wjg.v20.i44.16726
Boceprevir is highly effective in treatment-experienced hepatitis C virus-positive genotype-1 menopausal women
Abstract
Aim: To investigate the safety/efficacy of Boceprevir-based triple therapy in hepatitis C virus (HCV)-G1 menopausal women who were historic relapsers, partial-responders and null-responders.
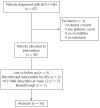
Methods: In this single-assignment, unblinded study, we treated fifty-six menopausal women with HCV-G1, 46% F3-F4, and previous PEG-α/RBV failure (7% null, 41% non-responder, and 52% relapser) with 4 wk lead-in with PEG-IFNα2b/RBV followed by PEG-IFNα2b/RBV+Boceprevir for 32 wk, with an additional 12 wk of PEG-IFN-α-2b/RBV if patients were HCV-RNA-positive by week 8. In previous null-responders, 44 wk of triple therapy was used. The primary objective of retreatment was to verify whether a sustained virological response (SVR) (HCV RNA undetectable at 24 wk of follow-up) rate of at least 20% could be obtained. The secondary objective was the evaluation of the percent of patients with negative HCV RNA at week 4 (RVR), 8 (RVR BOC), 12 (EVR), or at the end-of-treatment (ETR) that reached SVR. To assess the relationship between SVR and clinical and biochemical parameters, multiple logistic regression analysis was used.
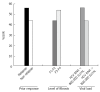
Results: After lead-in, only two patients had RVR; HCV-RNA was unchanged in all but 62% who had ≤ 1 log10 decrease. After Boceprevir, HCV RNA became undetectable at week 8 in 32/56 (57.1%) and at week 12 in 41/56 (73.2%). Of these, 53.8% and 52.0%, respectively, achieved SVR. Overall, SVR was obtained in 25/56 (44.6%). SVR was achieved in 55% previous relapsers vs. 41% non-responders (P = 0.250), in 44% F0-F2 vs 54% F3-F4 (P = 0.488), and in 11/19 (57.9%) of patients with cirrhosis. At univariate analysis for baseline predictors of SVR, only previous response to antiviral therapy (OR = 2.662, 95%CI: 0.957-6.881, P = 0.043), was related with SVR. When considering "on treatment" factors, 1 log10 HCV RNA decline at week 4 (3.733, 95%CI: 1.676-12.658, P = 0.034) and achievement of RVR BOC (7.347, 95%CI: 2.156-25.035, P = 0.001) were significantly related with the SVR, although RVR BOC only (6.794, 95%CI: 1.596-21.644, P = 0.010) maintained significance at multivariate logistic regression analysis. Anemia and neutropenia were managed with Erythropoietin and Filgrastim supplementation, respectively. Only six patients discontinued therapy.
Conclusion: Boceprevir obtained high SVR response independent of previous response, RVR or baseline fibrosis or cirrhosis. RVR BOC was the only independent predictor of SVR.
Trial registration: ClinicalTrials.gov NCT01457937.
Keywords: Genotype 1; Hepatitis C virus treatment; Menopause; Pegylated Interferon; Viral Hepatitis.
Figures
References
-
- Floreani A, Cazzagon N, Boemo DG, Baldovin T, Baldo V, Egoue J, Antoniazzi S, Minola E. Female patients in fertile age with chronic hepatitis C, easy genotype, and persistently normal transaminases have a 100% chance to reach a sustained virological response. Eur J Gastroenterol Hepatol. 2011;23:997–1003. - PubMed
-
- de Torres M, Poynard T. Risk factors for liver fibrosis progression in patients with chronic hepatitis C. Ann Hepatol. 2003;2:5–11. - PubMed
-
- Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008;134:1699–1714. - PubMed
-
- Estrabaud E, Vidaud M, Marcellin P, Asselah T. Genomics and HCV infection: progression of fibrosis and treatment response. J Hepatol. 2012;57:1110–1125. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

