Palliative effects and adverse events of strontium-89 for prostate cancer patients with bone metastasis
- PMID: 25469306
- PMCID: PMC4251176
- DOI: 10.3892/mco.2014.449
Palliative effects and adverse events of strontium-89 for prostate cancer patients with bone metastasis
Abstract
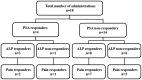
The aim of the present study was to evaluate the palliative effects and adverse events of strontium-89 (Sr-89) in patients with bone metastasis from prostate cancer. A total of 18 patients with prostate cancer and painful bone metastases, as diagnosed on bone scintigraphy, who were treated with Sr-89 at the National Kyushu Cancer Center between February, 2008 and April, 2014 were reviewed. Of the 18 subjects, 13 (72.2%) achieved a pain response, whereas 5 were classified as pain non-responders (27.8%). According to a logistic regression analysis, the pre-administration characteristics, including age, prostate-specific antigen (PSA), alkaline phosphatase (ALP), history of bone-modifying agent administration, opioid use or palliative radiation therapy, time after the combined androgen blockade nadir and time since the pain onset, were not found to be significant predictors of the pain response. Similarly, the post-administration characteristics, including pain flares and the PSA and ALP response, were not found to be significant predictors of the pain response. Although no patients exhibited leukocyte toxicities, 2 patients experienced myelosuppression, involving anemia and thrombocytopenia, requiring transfusion of red cell or platelet concentrate following Sr-89 treatment. Of the 18 patients, 5 (27.8%) reported pain flares, all of whom were successfully treated with rescue drugs alone. According to the logistic regression analysis, of the pre-administration characteristics, only ALP was identified as a significant predictor of bone marrow suppression in the univariate and multivariate analyses (P=0.006). Therefore, Sr-89 treatment was found to be effective in ameliorating bone pain associated with metastasis from prostate cancer. Although it is difficult to identify the patients who will receive pain relief prior to Sr-89 administration, this drug should be administered during the early stages due to the potential for bone marrow suppression in patients with high ALP levels.
Keywords: Japanese; adverse events; bone metastasis; palliative effects; prostate cancer; strontium-89.
Figures
Similar articles
-
EORTC QLQ-BM22 and QLQ-C30 quality of life scores in patients with painful bone metastases of prostate cancer treated with strontium-89 radionuclide therapy.Ann Nucl Med. 2012 Jul;26(6):485-91. doi: 10.1007/s12149-012-0598-z. Epub 2012 Apr 3. Ann Nucl Med. 2012. PMID: 22477263 Clinical Trial.
-
Strontium-89 for prostate cancer with bone metastases: the potential of cancer control and improvement of overall survival.Ann Nucl Med. 2014 Jan;28(1):11-6. doi: 10.1007/s12149-013-0775-8. Epub 2013 Oct 15. Ann Nucl Med. 2014. PMID: 24127064
-
Predictors of bone metastasis in pre-treatment staging of asymptomatic treatment-naïve patients with prostate cancer.Rev Esp Med Nucl Imagen Mol. 2013 Sep-Oct;32(5):286-9. doi: 10.1016/j.remn.2013.01.002. Epub 2013 Mar 9. Rev Esp Med Nucl Imagen Mol. 2013. PMID: 23478119 Clinical Trial.
-
Strontium 89 therapy for the palliation of pain due to osseous metastases.JAMA. 1995 Aug 2;274(5):420-4. JAMA. 1995. PMID: 7542352 Review.
-
Strontium chloride Sr 89 for treating pain from metastatic bone disease.Am J Health Syst Pharm. 1995 Oct 15;52(20):2189-95. doi: 10.1093/ajhp/52.20.2189. Am J Health Syst Pharm. 1995. PMID: 8564588 Review.
Cited by
-
Metastatic Spread in Prostate Cancer Patients Influencing Radiotherapy Response.Front Oncol. 2021 Mar 4;10:627379. doi: 10.3389/fonc.2020.627379. eCollection 2020. Front Oncol. 2021. PMID: 33747899 Free PMC article. Review.
-
Ra-223 Treatment for Bone Metastases in Castrate-Resistant Prostate Cancer: Practical Management Issues for Patient Selection.Am J Clin Oncol. 2019 Apr;42(4):399-406. doi: 10.1097/COC.0000000000000528. Am J Clin Oncol. 2019. PMID: 30844849 Free PMC article. Review.
-
Strontium-89 plus zoledronic acid versus zoledronic acid for patients with painful bone metastatic breast cancer.J Bone Miner Metab. 2022 Nov;40(6):998-1006. doi: 10.1007/s00774-022-01366-y. Epub 2022 Aug 30. J Bone Miner Metab. 2022. PMID: 36042056 Clinical Trial.
-
Exploiting bone niches: progression of disseminated tumor cells to metastasis.J Clin Invest. 2021 Mar 15;131(6):e143764. doi: 10.1172/JCI143764. J Clin Invest. 2021. PMID: 33720051 Free PMC article. Review.
-
A comparative evaluation of calix[4]arene-1,3-crown-6 as a ligand for selected divalent cations of radiopharmaceutical interest.RSC Adv. 2019 Oct 10;9(55):32357-32366. doi: 10.1039/c9ra07293d. eCollection 2019 Oct 7. RSC Adv. 2019. PMID: 35530789 Free PMC article.
References
-
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. - PubMed
-
- Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–1594. - PubMed
-
- Zekri J, Ahmed N, Coleman RE, Hancock BW. The skeletal metastatic complications of renal cell carcinoma. Int J Oncol. 2001;19:379–382. - PubMed
-
- Sciuto R, Festa A, Rea S, Pasqualoni R, Bergomi S, Petrilli G, Maini CL. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J Nucl Med. 2002;43:79–86. - PubMed
-
- Bolger JJ, Dearnaley DP, Kirk D, et al. Strontium-89 (Metastron) versus external beam radiotherapy in patients with painful bone metastases secondary to prostatic cancer: preliminary report of a multicenter trial. UK Metastron Investigators Group. Semin Oncol. 1993;20:32–33. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous