Keeping older muscle “young” through dietary protein and physical activity
- PMID: 25469405
- PMCID: PMC4188243
- DOI: 10.3945/an.113.005405
Keeping older muscle “young” through dietary protein and physical activity
Abstract
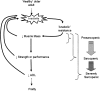
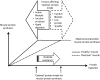
Sarcopenia is characterized by decreases in both muscle mass and muscle function. The loss of muscle mass, which can precede decrements in muscle function, is ultimately rooted in an imbalance between the rates of muscle protein synthesis and breakdown that favors a net negative balance (i.e., synthesis < breakdown). A preponderance of evidence highlights a blunted muscle protein synthetic response to dietary protein, commonly referred to as “anabolic resistance,” as a major underlying cause of the insipid loss of muscle with age. Dietary strategies to overcome this decreased dietary amino acid sensitivity include the ingestion of leucine-enriched, rapidly digested proteins and/or greater protein ingestion in each main meal to maximally stimulate muscle anabolism. Anabolic resistance is also a hallmark of a sedentary lifestyle at any age. Given that older adults may be more likely to experience periods of reduced activity (either voluntarily or through acute illness), it is proposed that inactivity is the precipitating factor in the development of anabolic resistance and the subsequent progression from healthy aging to frailty. However, even acute bouts of activity can restore the sensitivity of older muscle to dietary protein. Provided physical activity is incorporated into the daily routine, muscle in older adults should retain its capacity for a robust anabolic response to dietary protein comparable to that in their younger peers. Therefore, through its ability to “make nutrition better,” physical activity should be viewed as a vital component to maintaining muscle mass and function with age.
Conflict of interest statement
Author disclosure: D. R. Moore, no conflict of interest.
Figures
References
-
- Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 2005;82:941–8 - PubMed
-
- Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 2004;79:613–8 - PubMed
-
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–56 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

