The domestication of ortho-quinone methides
- PMID: 25469551
- PMCID: PMC4270411
- DOI: 10.1021/ar500330x
The domestication of ortho-quinone methides
Abstract
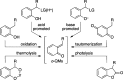
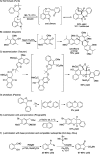
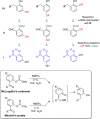
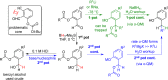
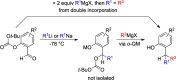
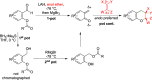
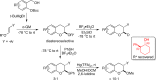
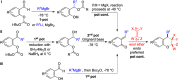
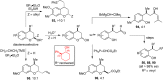
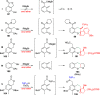
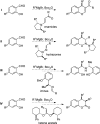
CONSPECTUS: An ortho-quinone methide (o-QM) is a highly reactive chemical motif harnessed by nature for a variety of purposes. Given its extraordinary reactivity and biological importance, it is surprising how few applications within organic synthesis exist. We speculate that their widespread use has been slowed by the complications that surround the preparation of their precursors, the harsh generation methods, and the omission of this stratagem from computer databases due to its ephemeral nature. About a decade ago, we discovered a mild anionic triggering procedure to generate transitory o-QMs at low temperature from readily available salicylaldehydes, particularly OBoc derivatives. This novel reaction cascade included both the o-QM formation and the subsequent consumption reaction. The overall transformation was initiated by the addition of the organometallic reagent, usually a Grignard reagent, which resulted in the formation of a benzyloxy alkoxide. Boc migration from the neighboring phenol produced a magnesium phenoxide that we supposed underwent β-elimination of the transferred Boc residue to form an o-QM for immediate further reactions. Moreover, the cascade proved controllable through careful manipulation of metallic and temperature levers so that it could be paused, stopped, or restarted at various intermediates and stages. This new level of domestication enabled us to deploy o-QMs for the first time in a range of applications including diastereocontrolled reactions. This sequence ultimately could be performed in either multipot or single pot processes. The subsequent reaction of the fleeting o-QM intermediates included the 1,4-conjugate additions that led to unbranched or branched ortho-alkyl substituted phenols and Diels-Alder reactions that provided 4-unsubstituted or 4-substituted benzopyrans and chroman ketals. The latter cycloadducts were obtained for the first time with outstanding diastereocontrol. In addition, the steric effects of the newly created stereocenters in subsequent reactions of chroman ketals and acetals were studied and proved predictable. Through the use of a chiral auxiliary, Diels-Alder products were deployed in numerous enantioselective reactions including several complex natural products syntheses. In this Account, we summarize our efforts, which we hope have contributed to the synthetic renaissance for this venerable species.
Figures
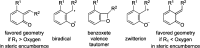
References
-
- Pathak T. P.; Sigman M. S. Applications of ortho-Quinone Methide Intermediates in Catalysis and Asymmetric Synthesis. J. Org. Chem. 2011, 76, 9210–9215. - PMC - PubMed
- Stokes S. M.; Ding F.; Smith P. L.; Keane J. M.; Kopach M. E.; Jervis R.; Sabat M.; Harman W. D. Formation of o-Quinone Methides from Dihapto-Coordinated Phenols and Their Controlled Release from a Transition Metal to Generate Chromans. Organometallics 2003, 22, 4170–4171.
-
- Alden-Danforth E.; Scerba M. T.; Lectka T. Asymmetric Cycloadditions of o-Quinone Methides Employing Chiral Ammonium Fluoride Precatalysts. Org. Lett. 2008, 10, 4951–4953. - PMC - PubMed
- Wu B.; Chen M.-W.; Ye Z.-S.; Yu C.-B.; Zhou Y.-G. A Streamlined Synthesis of 2,3-Dihydrobenzofurans via the ortho-Quinone Methides Generated from 2-Alkyl-Substituted Phenols. Adv. Synth. Catal. 2014, 356, 383–387.
-
- Luan Y.; Schaus S. Enantioselective Addition of Boronates to o-Quinone Methides Catalyzed by Chiral Biphenols. J. Am. Chem. Soc. 2012, 134, 19965–19968. - PMC - PubMed
- Izquierdo J.; Oreu A.; Scheidt K. A. A Dual Lewis Base Activation Strategy for Enantioselective Carbene-Catalyzed Annulations. J. Am. Chem. Soc. 2013, 135, 10634–10637. - PMC - PubMed
-
- Fries K.; Kann K. I. Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsproducte, Pseudobromide und Pseudochloride. Ueber o-Pseudohalogenide und o-Methylenchinone aus o-Oxymesitylalkohol. Liebigs Ann. Chem. 1907, 353, 335–356.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

