A reproducible and translatable model of focal ischemia in the visual cortex of infant and adult marmoset monkeys
- PMID: 25469561
- PMCID: PMC8029183
- DOI: 10.1111/bpa.12129
A reproducible and translatable model of focal ischemia in the visual cortex of infant and adult marmoset monkeys
Abstract
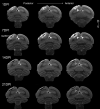
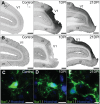
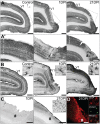
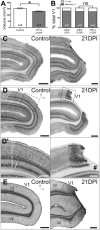
Models of ischemic brain injury in the nonhuman primate (NHP) are advantageous for investigating mechanisms of central nervous system (CNS) injuries and testing of new therapeutic strategies. However, issues of reproducibility and survivability persist in NHP models of CNS injuries. Furthermore, there are currently no pediatric NHP models of ischemic brain injury. Therefore, we have developed a NHP model of cortical focal ischemia that is highly reproducible throughout life to enable better understanding of downstream consequences of injury. Posterior cerebral arterial occlusion was induced through intracortical injections of endothelin-1 in adult (n = 5) and neonatal (n = 3) marmosets, followed by magnetic resonance imaging (MRI), histology and immunohistochemistry. MRI revealed tissue hyperintensity at the lesion site at 1-7 days followed by isointensity at 14-21 days. Peripheral macrophage and serum albumin infiltration was detected at 1 day, persisting at 21 days. The proportional loss of total V1 as a result of infarction was consistent in adults and neonates. Minor hemorrhagic transformation was detected at 21 days at the lesion core, while neovascularization was detected in neonates, but not in adults. We have developed a highly reproducible and survivable model of focal ischemia in the adult and neonatal marmoset primary visual cortex, demonstrating similar downstream anatomical and cellular pathology to those observed in post-ischemic humans.
Keywords: MRI; cerebral ischemia; endothelin‐1; neural degeneration; primary visual cortex; primate.
© 2014 International Society of Neuropathology.
Conflict of interest statement
None.
Figures




References
-
- Aldrich MS, Alessi AG, Beck RW, Gilman S (1987) Cortical blindness: etiology, diagnosis, and prognosis. Ann Neurol 21:149–158. - PubMed
-
- Allen LM, Hasso AN, Handwerker J, Farid H (2012) Sequence‐specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 32:1285–1297, discussion 97–9. - PubMed
-
- Anderson V, Spencer‐Smith M, Wood A (2011) Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 134(Pt 8):2197–2221. - PubMed
-
- Ang LC, Munoz DG, Shul D, George DH (1991) SMI‐32 immunoreactivity in human striate cortex during postnatal development. Brain Res Dev Brain Res 61:103–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

