HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes
- PMID: 25469638
- PMCID: PMC4254744
- DOI: 10.1371/journal.pone.0113799
HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes
Abstract
Objective: Spontaneous preterm birth (PTB) and preterm prelabor rupture of membranes (pPROM) are major pregnancy complications often associated with a fetal inflammatory response. Biomolecular markers of this fetal inflammatory response to both infectious and non-infectious risk factors and their contribution to PTB and pPROM mechanism are still unclear. This study examined fetal membrane production, activation and mechanistic properties of high mobility group box 1 (HMGB1) as a contributor of the non-infectious fetal inflammatory response.
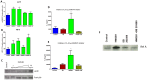
Materials and methods: HMGB1 transcripts and active HMGB1 were profiled in fetal membranes and amniotic fluids collected from PTB and normal term birth. In vitro, normal term not in labor fetal membranes were exposed to lipopolysaccharide (LPS) and water soluble cigarette smoke extract (CSE). HMGB1-transcripts and its protein concentrations were documented by RT-PCR and ELISA. Recombinant HMGB1 treated membranes and media were subjected to RT-PCR for HMGB1 receptors, mitogen activated protein kinase pathway analysis, cytokine levels, and Western blot for p38MAPK.
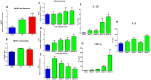
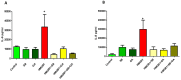
Results: HMGB1 expression and its active forms were higher in PTB and pPROM than normal term membranes and amniotic fluid samples. Both LPS and CSE enhanced HMGB1 expression and release in vitro. Fetal membrane exposure to HMGB1 resulted in increased expression of TLR2 and 4 and dose-dependent activation of p38MAPK-mediated inflammation.
Conclusions: HMGB1 increase by fetal membrane cells in response to either oxidative stress or infection can provide a positive feedback loop generating non-infectious inflammatory activation. Activation of p38MAPK by HMGB1 promotes development of the senescence phenotype and senescence associated sterile inflammation. HMGB1 activity is an important regulator of the fetal inflammatory response regardless of infection.
Conflict of interest statement
Figures



References
-
- Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, et al. (2014) Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 210:125. - PubMed
-
- Menon R, Fortunato SJ (2009) Distinct pathophysiologic pathways induced by in vitro infection and cigarette smoke in normal human fetal membranes. Am J Obstet Gynecol 200:334–338. - PubMed
-
- Bartling B, Fuchs C, Somoza V, Niemann B, Silber RE, et al. (2007) Lung level of HMBG1 is elevated in response to advanced glycation end product-enriched food in vivo. Mol Nutr Food Res 51:479–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

