The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention
- PMID: 25469720
- PMCID: PMC4300282
- DOI: 10.1111/vox.12210
The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention
Abstract
Background and objectives: Repeated blood donation produces iron deficiency. Changes in dietary iron intake do not prevent donation-induced iron deficiency. Prolonging the interdonation interval or using oral iron supplements can mitigate donation-induced iron deficiency. The most effective operational methods for reducing iron deficiency in donors are unknown.
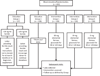
Materials and methods: 'Strategies To Reduce Iron Deficiency' (STRIDE) was a two-year, randomized, placebo-controlled study in blood donors. 692 donors were randomized into one of two educational groups or one of three interventional groups. Donors randomized to educational groups either received letters thanking them for donating, or, suggesting iron supplements or delayed donation if they had low ferritin. Donors randomized to interventional groups either received placebo, 19-mg or 38-mg iron pills.
Results: Iron deficient erythropoiesis was present in 52·7% of males and 74·6% of females at enrolment. Adverse events within 60 days of enrolment were primarily mild gastrointestinal symptoms (64%). The incidence of de-enrolment within 60 days was more common in the interventional groups than in the educational groups (P = 0·002), but not more common in those receiving iron than placebo (P = 0·68).
Conclusion: The prevalence of iron deficient erythropoiesis in donors enrolled in the STRIDE study is comparable to previously described cohorts of regular blood donors. De-enrolment within 60 days was higher for donors receiving tablets, although no more common in donors receiving iron than placebo.
Keywords: anaemia; blood donation; ferritin; iron deficiency.
© 2014 International Society of Blood Transfusion.
Conflict of interest statement
AEM has received honoraria from Siemens. The other authors have no competing interests.
Figures
References
-
- Finch CA, Cook JD, Labbe RF, et al. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood. 1977;50:441–447. - PubMed
-
- Simon TL, Garry PJ, Hooper EM. Iron stores in blood donors. JAMA. 1981;245:2038–2043. - PubMed
-
- Page EA, Coppock JE, Harrison JF. Study of iron stores in regular plate-letpheresis donors. Transfus Med. 2010;20:22–29. - PubMed
-
- Beutler E, Larsh SE, Gurney CW. Iron therapy in chronically fatigued, nona-nemic women: a double-blind study. Ann Intern Med. 1960;52:378–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical