Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors
- PMID: 25469886
- PMCID: PMC4254997
- DOI: 10.1371/journal.pone.0114249
Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors
Abstract
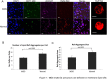
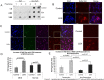
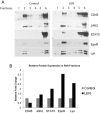
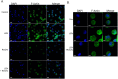
Anemia remains the principal management challenge for patients with lower risk Myelodysplastic Syndromes (MDS). Despite appropriate cytokine production and cellular receptor display, erythropoietin receptor (EpoR) signaling is impaired. We reported that EpoR signaling is dependent upon receptor localization within lipid raft microdomains, and that disruption of raft integrity abolishes signaling capacity. Here, we show that MDS erythroid progenitors display markedly diminished raft assembly and smaller raft aggregates compared to normal controls (p = 0.005, raft number; p = 0.023, raft size). Because lenalidomide triggers raft coalescence in T-lymphocytes promoting immune synapse formation, we assessed effects of lenalidomide on raft assembly in MDS erythroid precursors and UT7 cells. Lenalidomide treatment rapidly induced lipid raft formation accompanied by EpoR recruitment into raft fractions together with STAT5, JAK2, and Lyn kinase. The JAK2 phosphatase, CD45, a key negative regulator of EpoR signaling, was displaced from raft fractions. Lenalidomide treatment prior to Epo stimulation enhanced both JAK2 and STAT5 phosphorylation in UT7 and primary MDS erythroid progenitors, accompanied by increased STAT5 DNA binding in UT7 cells, and increased erythroid colony forming capacity in both UT7 and primary cells. Raft induction was associated with F-actin polymerization, which was blocked by Rho kinase inhibition. These data indicate that deficient raft integrity impairs EpoR signaling, and provides a novel strategy to enhance EpoR signal fidelity in non-del(5q) MDS.
Conflict of interest statement
Figures


References
-
- Hoefsloot LH, van Amelsvoort MP, Broeders LC, van der Plas DC, van Lom K, et al. (1997) Erythropoietin-induced activation of STAT5 is impaired in the myelodysplastic syndrome. Blood 89:1690–1700. - PubMed
-
- Fuhler GM, Blom NR, Coffer PJ, Drayer AL, Vellenga E (2007) The reduced GM-CSF priming of ROS production in granulocytes from patients with myelodysplasia is associated with an impaired lipid raft formation. Journal of leukocyte biology 81:449–457. - PubMed
-
- Xavier R, Brennan T, Li Q, McCormack C, Seed B (1998) Membrane compartmentation is required for efficient T cell activation. Immunity 8:723–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

