Interception of host angiogenic signalling limits mycobacterial growth
- PMID: 25470057
- PMCID: PMC4312197
- DOI: 10.1038/nature13967
Interception of host angiogenic signalling limits mycobacterial growth
Abstract
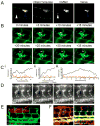
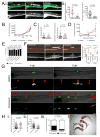
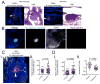
Pathogenic mycobacteria induce the formation of complex cellular aggregates called granulomas that are the hallmark of tuberculosis. Here we examine the development and consequences of vascularization of the tuberculous granuloma in the zebrafish-Mycobacterium marinum infection model, which is characterized by organized granulomas with necrotic cores that bear striking resemblance to those of human tuberculosis. Using intravital microscopy in the transparent larval zebrafish, we show that granuloma formation is intimately associated with angiogenesis. The initiation of angiogenesis in turn coincides with the generation of local hypoxia and transcriptional induction of the canonical pro-angiogenic molecule Vegfaa. Pharmacological inhibition of the Vegf pathway suppresses granuloma-associated angiogenesis, reduces infection burden and limits dissemination. Moreover, anti-angiogenic therapies synergize with the first-line anti-tubercular antibiotic rifampicin, as well as with the antibiotic metronidazole, which targets hypoxic bacterial populations. Our data indicate that mycobacteria induce granuloma-associated angiogenesis, which promotes mycobacterial growth and increases spread of infection to new tissue sites. We propose the use of anti-angiogenic agents, now being used in cancer regimens, as a host-targeting tuberculosis therapy, particularly in extensively drug-resistant disease for which current antibiotic regimens are largely ineffective.
Conflict of interest statement
The authors have no competing financial interests.
Figures








References
-
- Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. - PubMed
-
- Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–366. - PubMed
-
- Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs. 1997;54:679–708. - PubMed
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29:15–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

