The chemistry of curcumin: from extraction to therapeutic agent
- PMID: 25470276
- PMCID: PMC6270789
- DOI: 10.3390/molecules191220091
The chemistry of curcumin: from extraction to therapeutic agent
Abstract
Curcumin, a pigment from turmeric, is one of the very few promising natural products that has been extensively investigated by researchers from both the biological and chemical point of view. While there are several reviews on the biological and pharmacological effects of curcumin, chemistry reviews are comparatively scarcer. In this article, an overview of different aspects of the unique chemistry research on curcumin will be discussed. These include methods for the extraction from turmeric, laboratory synthesis methods, chemical and photochemical degradation and the chemistry behind its metabolism. Additionally other chemical reactions that have biological relevance like nucleophilic addition reactions, and metal chelation will be discussed. Recent advances in the preparation of new curcumin nanoconjugates with metal and metal oxide nanoparticles will also be mentioned. Directions for future investigations to be undertaken in the chemistry of curcumin have also been suggested.
Conflict of interest statement
The author declares no conflict of interest.
Figures


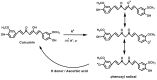
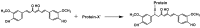


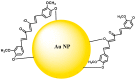
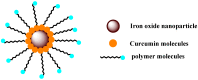
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

