The Dbf4-Cdc7 kinase promotes Mcm2-7 ring opening to allow for single-stranded DNA extrusion and helicase assembly
- PMID: 25471369
- PMCID: PMC4294486
- DOI: 10.1074/jbc.M114.608232
The Dbf4-Cdc7 kinase promotes Mcm2-7 ring opening to allow for single-stranded DNA extrusion and helicase assembly
Retraction in
-
The Dbf4-Cdc7 kinase promotes Mcm2-7 ring opening to allow for single-stranded DNA extrusion and helicase assembly.J Biol Chem. 2017 Jun 16;292(24):10320. doi: 10.1074/jbc.A114.608232. J Biol Chem. 2017. PMID: 28623198 Free PMC article. No abstract available.
Abstract
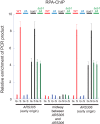
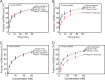
The replication fork helicase in eukaryotes is composed of Cdc45, Mcm2-7, and GINS (CMG). The Dbf4-Cdc7 kinase phosphorylates Mcm2 in vitro, but the in vivo role for Dbf4-Cdc7 phosphorylation of Mcm2 is unclear. We find that budding yeast Dbf4-Cdc7 phosphorylates Mcm2 in vivo under normal conditions during S phase. Inhibiting Dbf4-Cdc7 phosphorylation of Mcm2 confers a dominant-negative phenotype with a severe growth defect. Inhibiting Dbf4-Cdc7 phosphorylation of Mcm2 under wild-type expression conditions also results in impaired DNA replication, substantially decreased single-stranded formation at an origin, and markedly disrupted interaction between GINS and Mcm2-7 during S phase. In vitro, Dbf4-Cdc7 kinase (DDK) phosphorylation of Mcm2 substantially weakens the interaction between Mcm2 and Mcm5, and Dbf4-Cdc7 phosphorylation of Mcm2 promotes Mcm2-7 ring opening. The extrusion of ssDNA from the central channel of Mcm2-7 triggers GINS attachment to Mcm2-7. Thus, Dbf4-Cdc7 phosphorylation of Mcm2 may open the Mcm2-7 ring at the Mcm2-Mcm5 interface, allowing for single-stranded DNA extrusion and subsequent GINS assembly with Mcm2-7.
Keywords: DNA Helicase; DNA Replication; DNA-binding Protein; Initiation; Kinase; Phosphorylation; Protein-Protein Interaction.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Pacek M., Tutter A. V., Kubota Y., Takisawa H., Walter J. C. (2006) Localization of MCM2-7, Cdc45, and GINS to the site of DNA unwinding during eukaryotic DNA replication. Mol. Cell. 21, 581–587 - PubMed
-
- Gambus A., Jones R. C., Sanchez-Diaz A., Kanemaki M., van Deursen F., Edmondson R. D., Labib K. (2006) GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 8, 358–366 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

