Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state
- PMID: 25471570
- PMCID: PMC6608488
- DOI: 10.1523/JNEUROSCI.4622-13.2014
Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state
Abstract
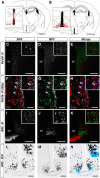
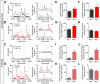
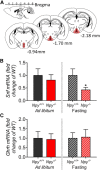
The hypothalamic NPY system plays an important role in regulating food intake and energy expenditure. Different biological actions of NPY are assigned to NPY receptor subtypes. Recent studies demonstrated a close relationship between food intake and growth hormone (GH) secretion; however, the mechanism through which endogenous NPY modulates GH release remains unknown. Moreover, conclusive evidence demonstrating a role for NPY and Y-receptors in regulating the endogenous pulsatile release of GH does not exist. We used genetically modified mice (germline Npy, Y1, and Y2 receptor knock-out mice) to assess pulsatile GH secretion under both fed and fasting conditions. Deletion of NPY did not impact fed GH release; however, it reversed the fasting-induced suppression of pulsatile GH secretion. The recovery of GH secretion was associated with a reduction in hypothalamic somatotropin release inhibiting factor (Srif; somatostatin) mRNA expression. Moreover, observations revealed a differential role for Y1 and Y2 receptors, wherein the postsynaptic Y1 receptor suppresses GH secretion in fasting. In contrast, the presynaptic Y2 receptor maintains normal GH output under long-term ad libitum-fed conditions. These data demonstrate an integrated neural circuit that modulates GH release relative to food intake, and provide essential information to address the differential roles of Y1 and Y2 receptors in regulating the release of GH under fed and fasting states.
Keywords: NPY; NPY-receptors; fasting; feeding; growth hormone; somatostatin.
Copyright © 2014 the authors 0270-6474/14/3416309-11$15.00/0.
Figures





References
-
- Adams EF, Venetikou MS, Woods CA, Lacoumenta S, Burrin JM. Neuropeptide Y directly inhibits growth hormone secretion by human pituitary somatotropic tumours. Acta Endocrinol. 1987;115:149–154. - PubMed
-
- Balasubramaniam A, Joshi R, Su C, Friend LA, James JH. Neuropeptide Y (NPY) Y2 receptor-selective agonist inhibits food intake and promotes fat metabolism in mice: combined anorectic effects of Y2 and Y4 receptor-selective agonists. Peptides. 2007;28:235–240. doi: 10.1016/j.peptides.2006.08.041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
