Piezo2 is the major transducer of mechanical forces for touch sensation in mice
- PMID: 25471886
- PMCID: PMC4380172
- DOI: 10.1038/nature13980
Piezo2 is the major transducer of mechanical forces for touch sensation in mice
Abstract
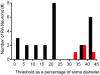
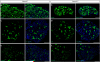
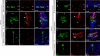
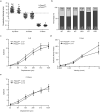
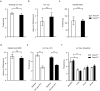
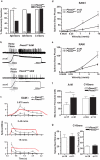
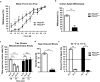
The sense of touch provides critical information about our physical environment by transforming mechanical energy into electrical signals. It is postulated that mechanically activated cation channels initiate touch sensation, but the identity of these molecules in mammals has been elusive. Piezo2 is a rapidly adapting, mechanically activated ion channel expressed in a subset of sensory neurons of the dorsal root ganglion and in cutaneous mechanoreceptors known as Merkel-cell-neurite complexes. It has been demonstrated that Merkel cells have a role in vertebrate mechanosensation using Piezo2, particularly in shaping the type of current sent by the innervating sensory neuron; however, major aspects of touch sensation remain intact without Merkel cell activity. Here we show that mice lacking Piezo2 in both adult sensory neurons and Merkel cells exhibit a profound loss of touch sensation. We precisely localize Piezo2 to the peripheral endings of a broad range of low-threshold mechanoreceptors that innervate both hairy and glabrous skin. Most rapidly adapting, mechanically activated currents in dorsal root ganglion neuronal cultures are absent in Piezo2 conditional knockout mice, and ex vivo skin nerve preparation studies show that the mechanosensitivity of low-threshold mechanoreceptors strongly depends on Piezo2. This cellular phenotype correlates with an unprecedented behavioural phenotype: an almost complete deficit in light-touch sensation in multiple behavioural assays, without affecting other somatosensory functions. Our results highlight that a single ion channel that displays rapidly adapting, mechanically activated currents in vitro is responsible for the mechanosensitivity of most low-threshold mechanoreceptor subtypes involved in innocuous touch sensation. Notably, we find that touch and pain sensation are separable, suggesting that as-yet-unknown mechanically activated ion channel(s) must account for noxious (painful) mechanosensation.
Figures
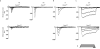




References
-
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi:10.1146/annurev.biophys.37.032807.125836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

