Systematic and transparent inclusion of ethical issues and recommendations in clinical practice guidelines: a six-step approach
- PMID: 25472446
- PMCID: PMC4265426
- DOI: 10.1186/s13012-014-0184-y
Systematic and transparent inclusion of ethical issues and recommendations in clinical practice guidelines: a six-step approach
Abstract
Background: Clinical practice guidelines (CPGs), a core tool to foster medical professionalism, differ widely in whether and how they address disease-specific ethical issues (DSEIs), and current manuals for CPG development are silent on this issue. The implementation of an explicit method faces two core challenges: first, it adds further complexity to CPG development and requires human and financial resources. Second, in contrast to the in-depth treatment of ethical issues that is standard in bioethics, the inclusion of DSEIs in CPGs need to be more pragmatic, reductive, and simplistic, but without rendering the resulting recommendations useless or insufficiently justified. This paper outlines a six-step approach, EthicsGuide, for the systematic and transparent inclusion of ethical issues and recommendations in CPGs.
Methods: The development of EthicsGuide is based on (a) methodological standards in evidence-based CPG development, (b) principles of bioethics, (c) research findings on how DSEIs are currently addressed in CPGs, and (d) findings from two proof-of-concept analyses of the EthicsGuide approach.
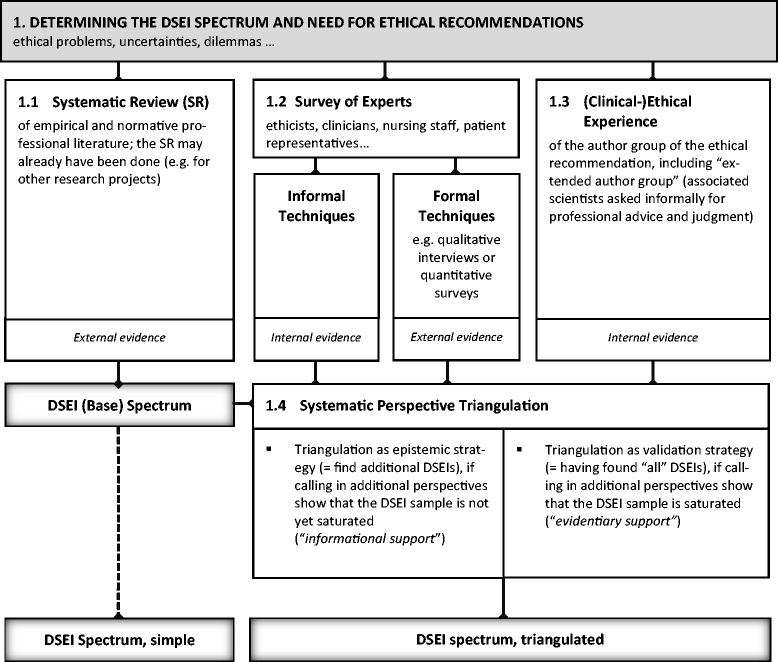
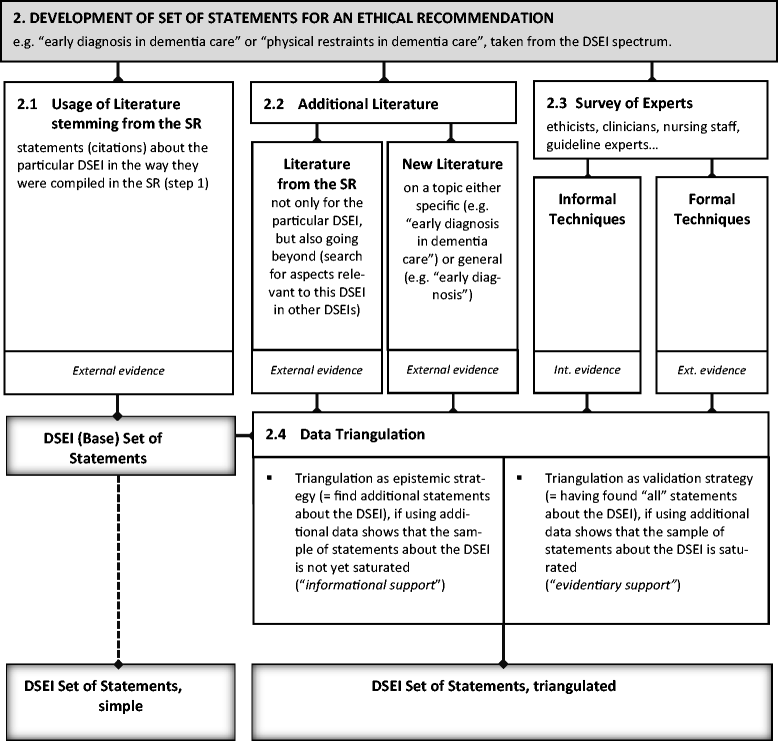
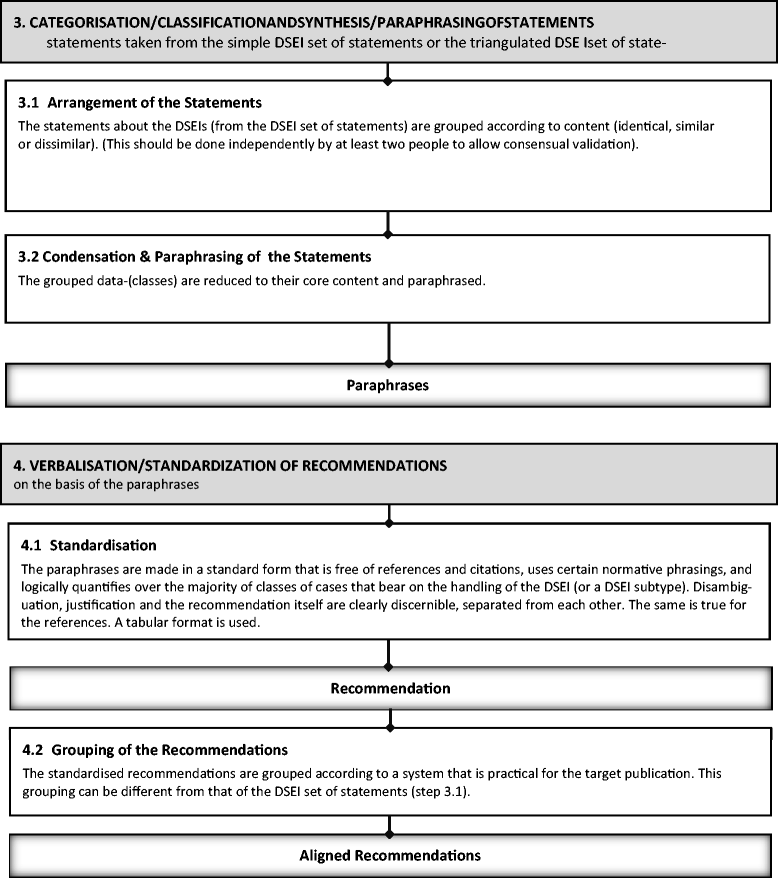
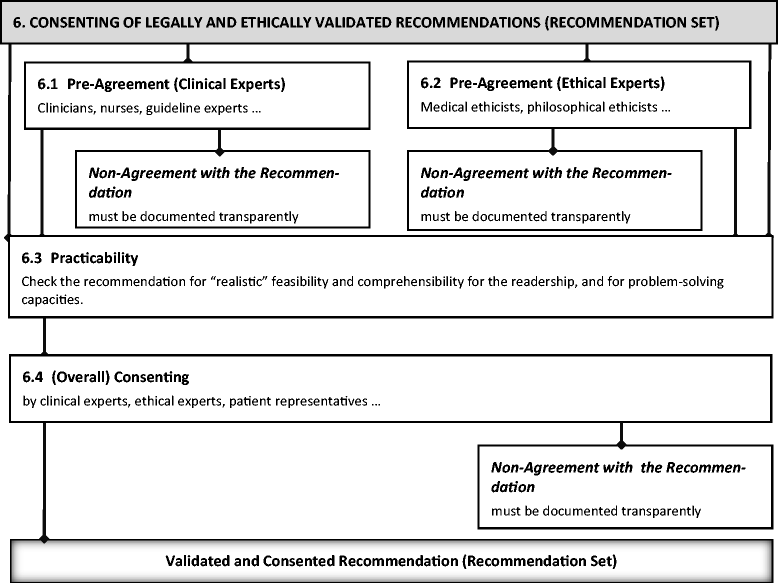
Results: The six steps are 1) determine the DSEI spectrum and the need for ethical recommendations; 2) develop statements on which to base ethical recommendations; 3) categorize, classify, condense, and paraphrase the statements; 4) write recommendations in a standard form; 5) validate and justify recommendations, making any necessary modifications; and 6) address consent. All six steps necessarily come into play when including DSEIs in CPGs.
Conclusions: If DSEIs are not explicitly addressed, they are unavoidably dealt with implicitly. We believe that as ethicists gain greater involvement in decision-making about health, personal rights, or economic issues, they should make their methods transparent and replicable by other researchers; and as ethical issues become more widely reflected in CPGs, CPG developers have to learn how to address them in a methodologically adequate way. The approach proposed should serve as a basis for further discussion on how to reach these goals. It breaks open the black box of what ethicists implicitly do when they develop recommendations. Further, interdisciplinary discussion and pilot tests are needed to explore the minimal requirements that guarantee a simplified procedure which is still acceptable and does not become mere window dressing.
Figures
References
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. - DOI - PMC - PubMed
-
- IOM: Clinical practice guidelines we can trust. Washington D.C.: National Academies Press, Institute of Medicine (IOM); 2011.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources