β2-adrenergic regulation of stress hyperglycemia following hemorrhage in the obese Zucker rat
- PMID: 25472607
- PMCID: PMC4332203
- DOI: 10.14814/phy2.12215
β2-adrenergic regulation of stress hyperglycemia following hemorrhage in the obese Zucker rat
Abstract
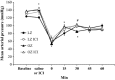
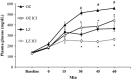
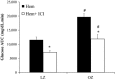
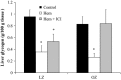
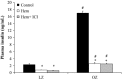
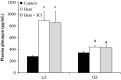
Stress hyperglycemia following trauma has been shown to potentiate morbidity and mortality. Glucose control in obese patients can be challenging due to insulin resistance. Thus, understanding the mechanisms for glucose generation following hemorrhage may provide important insights into alternative options for glycemic control in obesity. Obesity is characterized by elevated glycogen and increased hepatic β2-adrenergic activity, which play major roles in glucose production after hemorrhage. We hypothesized that, in obesity, hepatic glycogenolysis is enhanced during stress hyperglycemia due to increased hepatic β2-adrenoceptor activation. Hemorrhage was performed in conscious lean Zucker (LZ) and obese Zucker rats (OZ) by withdrawing 35% total blood volume over 10 min. Liver glycogen content and plasma levels of glucose, insulin, and glucagon were measured before and 1 h after hemorrhage. The hyperglycemic response was greater in OZ as compared to LZ, but glycogen content was similarly reduced in both groups. Subsequently, OZ had a greater fall in insulin compared to LZ. Glucagon levels were significantly increased 1 h after hemorrhage in LZ but not in OZ. To test the direct adrenergic effects on the liver after hemorrhage, we treated animals before hemorrhage with a selective β2-adrenoceptor antagonist, ICI-118,551 (ICI; 2 mg/kg/h, i.v.). After hemorrhage, ICI significantly reduced hyperglycemia in both LZ and OZ, independent of hormonal changes, but there was a significantly decreased hepatic glycogenolysis in OZ. These results suggest that the hemorrhage-induced hepatic glycogenolysis is likely glucagon-dependent in LZ, whereas the β2-adrenoceptor plays a greater role in OZ.
Keywords: Hemorrhage; hyperglycemia; obesity; β2 adrenoreceptor.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures
References
-
- Arinze I. J., Kawai Y. 1983. Adrenergic regulation of glycogenolysis in isolated guinea‐pig hepatocytes: evidence that beta 2‐receptors mediate catecholamine stimulation of glycogenolysis. Arch. Biochem. Biophys.; 225:196-202. - PubMed
-
- Atkinson R. L., Dahms W. T., Bray G. A., Sperling M. A. 1981. Adrenergic modulation of glucagon and insulin secretion in obese and lean humans. Horm. Metab. Res.; 13:249-253. - PubMed
-
- Basu R., Chandramouli V., Dicke B., Landau B., Rizza R. 2005. Obesity and type 2 diabetes impair insulin‐induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes; 54:1942-1948. - PubMed
-
- Begin‐Heick N. 1994. Liver beta‐adrenergic receptors, G proteins, and adenylyl cyclase activity in obesity‐diabetes syndromes. Am. J. Physiol.; 266:C1664-C1672. - PubMed
-
- van den Berghe G., Wouters P., Weekers F., Verwaest C., Bruyninckx F., Schetz M. 2001. Intensive insulin therapy in critically ill patients. N. Engl. J. Med.; 345:1359-1367. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

