TRPM7 regulates vascular endothelial cell adhesion and tube formation
- PMID: 25472964
- PMCID: PMC4329423
- DOI: 10.1152/ajpcell.00275.2013
TRPM7 regulates vascular endothelial cell adhesion and tube formation
Abstract
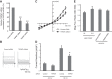
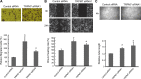
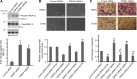
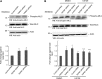
Transient receptor potential melastatin 7 (TRPM7) is a nonselective cation channel with an α-kinase domain in its COOH terminal, known to play a role in diverse physiological and pathological processes such as Mg2+ homeostasis, cell proliferation, and hypoxic neuronal injury. Increasing evidence suggests that TRPM7 contributes to the physiology/pathology of vascular systems. For example, we recently demonstrated that silencing TRPM7 promotes growth and proliferation and protects against hyperglycemia-induced injury in human umbilical vein endothelial cells (HUVECs). Here we investigated the potential effects of TRPM7 on morphology, adhesion, migration, and tube formation of vascular endothelial cells and the potential underlying mechanism. We showed that inhibition of TRPM7 function in HUVECs by silencing TRPM7 decreases the density of TRPM7-like current and cell surface area and inhibits cell adhesion to Matrigel. Silencing TRPM7 also promotes cell migration, wound healing, and tube formation. Further studies showed that the extracellular signal-regulated kinase (ERK) pathway is involved in the change of cell morphology and the increase in HUVEC migration induced by TRPM7 silencing. We also demonstrated that silencing TRPM7 enhances the phosphorylation of myosin light chain (MLC) in HUVECs, which might be involved in the enhancement of cell contractility and motility. Collectively, our data suggest that the TRPM7 channel negatively regulates the function of vascular endothelial cells. Further studies on the underlying mechanism may facilitate the development of the TRPM7 channel as a target for the therapeutic intervention of vascular diseases.
Keywords: HUVECs; TRPM7; adhesion; migration; tube formation.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell 115: 863–877, 2003. - PubMed
-
- Baldoli E, Maier JA. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis 15: 47–57, 2012. - PubMed
-
- Brahmbhatt AA, Klemke RL. ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J Biol Chem 278: 13016–13025, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

