Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial
- PMID: 25472980
- PMCID: PMC4323569
- DOI: 10.1161/CIRCULATIONAHA.114.011004
Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial
Abstract
Background: Drug-coated balloons (DCBs) have shown promise in improving the outcomes for patients with peripheral artery disease. We compared a paclitaxel-coated balloon with percutaneous transluminal angioplasty (PTA) for the treatment of symptomatic superficial femoral and popliteal artery disease.
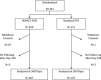
Methods and results: The IN.PACT SFA Trial is a prospective, multicenter, single-blinded, randomized trial in which 331 patients with intermittent claudication or ischemic rest pain attributable to superficial femoral and popliteal peripheral artery disease were randomly assigned in a 2:1 ratio to treatment with DCB or PTA. The primary efficacy end point was primary patency, defined as freedom from restenosis or clinically driven target lesion revascularization at 12 months. Baseline characteristics were similar between the 2 groups. Mean lesion length and the percentage of total occlusions for the DCB and PTA arms were 8.94 ± 4.89 and 8.81 ± 5.12 cm (P=0.82) and 25.8% and 19.5% (P=0.22), respectively. DCB resulted in higher primary patency versus PTA (82.2% versus 52.4%; P<0.001). The rate of clinically driven target lesion revascularization was 2.4% in the DCB arm in comparison with 20.6% in the PTA arm (P<0.001). There was a low rate of vessel thrombosis in both arms (1.4% after DCB and 3.7% after PTA [P=0.10]). There were no device- or procedure-related deaths and no major amputations.
Conclusions: In this prospective, multicenter, randomized trial, DCB was superior to PTA and had a favorable safety profile for the treatment of patients with symptomatic femoropopliteal peripheral artery disease.
Clinical trial registration url: http://www.clinicaltrials.gov. Unique Identifiers: NCT01175850 and NCT01566461.
Keywords: drug-eluting balloons; peripheral arterial disease; peripheral vascular diseases.
© 2014 The Authors.
Figures

References
-
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. - PubMed
-
- European Society of Cardiology (ESC), Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Röther J, Sievert H, van Sambeek M, Zeller T ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–2906. - PubMed
-
- Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G VIVA Physicians, Inc. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. doi: 10.1002/ccd.21104. - PubMed
-
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

