PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis
- PMID: 25473034
- PMCID: PMC5567784
- DOI: 10.1126/scitranslmed.3009501
PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis
Abstract
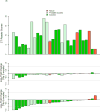
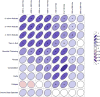
Definitive clinical trials of new chemotherapies for treating tuberculosis (TB) require following subjects until at least 6 months after treatment discontinuation to assess for durable cure, making these trials expensive and lengthy. Surrogate endpoints relating to treatment failure and relapse are currently limited to sputum microbiology, which has limited sensitivity and specificity. We prospectively assessed radiographic changes using 2-deoxy-2-[(18)F]-fluoro-D-glucose (FDG) positron emission tomography/computed tomography (PET/CT) at 2 and 6 months (CT only) in a cohort of subjects with multidrug-resistant TB, who were treated with second-line TB therapy for 2 years and then followed for an additional 6 months. CT scans were read semiquantitatively by radiologists and were computationally evaluated using custom software to provide volumetric assessment of TB-associated abnormalities. CT scans at 6 months (but not 2 months) assessed by radiologist readers were predictive of outcomes, and changes in computed abnormal volumes were predictive of drug response at both time points. Quantitative changes in FDG uptake 2 months after starting treatment were associated with long-term outcomes. In this cohort, some radiologic markers were more sensitive than conventional sputum microbiology in distinguishing successful from unsuccessful treatment. These results support the potential of imaging scans as possible surrogate endpoints in clinical trials of new TB drug regimens. Larger cohorts confirming these results are needed.
Copyright © 2014, American Association for the Advancement of Science.
Conflict of interest statement
Figures







References
-
- Horne DJ, Royce SE, Gooze L, Narita M, Hopewell PC, Nahid P, Steingart KR. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. The Lancet infectious diseases. 2010;10:387–394. doi: 10.1016/S1473-3099(10)70071-2. published online EpubJun. - DOI - PMC - PubMed
-
- Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, Gordin F, Horsburgh CR, Horton J, Khan A, Lahart C, Metchock B, Pachucki C, Stanton L, Vernon A, Villarino ME, Wang YC, Weiner M, Weis S C. Tuberculosis Trials. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–534. published online. - PubMed
-
- Johnson JL, Hadad DJ, Dietze R, Maciel EL, Sewali B, Gitta P, Okwera A, Mugerwa RD, Alcaneses MR, Quelapio MI, Tupasi TE, Horter L, Debanne SM, Eisenach KD, Boom WH. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. American journal of respiratory and critical care medicine. 2009;180:558–563. doi: 10.1164/rccm.200904-0536OC. published online EpubSep 15. - DOI - PMC - PubMed
-
- Phillips PP, Nunn AJ, Paton NI. Is a 4-month regimen adequate to cure patients with non-cavitary tuberculosis and negative cultures at 2 months? The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2013;17:807–809. doi: 10.5588/ijtld.12.0725. published online EpubJun. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

