Generation and evaluation of clade C simian-human immunodeficiency virus challenge stocks
- PMID: 25473043
- PMCID: PMC4338880
- DOI: 10.1128/JVI.03279-14
Generation and evaluation of clade C simian-human immunodeficiency virus challenge stocks
Abstract
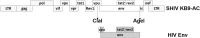
The development of a panel of mucosally transmissible simian-human immunodeficiency virus (SHIV) challenge stocks from multiple virus clades would facilitate preclinical evaluation of candidate HIV-1 vaccines and therapeutics. The majority of SHIV stocks that have been generated to date have been derived from clade B HIV-1 env sequences from viruses isolated during chronic infection and typically required serial animal-to-animal adaptation for establishing mucosal transmissibility and pathogenicity. To capture essential features of mucosal transmission of clade C viruses, we produced a series of SHIVs with early clade C HIV-1 env sequences from acutely HIV-1-infected individuals from South Africa. SHIV-327c and SHIV-327cRM expressed env sequences that were 99.7 to 100% identical to the original HIV-1 isolate and did not require in vivo passaging for mucosal infectivity. These challenge stocks infected rhesus monkeys efficiently by both intrarectal and intravaginal routes, replicated to high levels during acute infection, and established chronic setpoint viremia in 13 of 17 (76%) infected animals. The SHIV-327cRM challenge stock was also titrated for both single, high-dose intrarectal challenges and repetitive, low-dose intrarectal challenges in rhesus monkeys. These SHIV challenge stocks should facilitate the preclinical evaluation of vaccines and other interventions aimed at preventing clade C HIV-1 infection.
Importance: We describe the development of two related clade C SHIV challenge stocks. These challenge stocks should prove useful for preclinical testing of vaccines and other interventions aimed at preventing clade C HIV-1 infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Lu Y, Salvato MS, Pauza CD, Li J, Sodroski J, Manson K, Wyand M, Letvin N, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne LG, Roberts B. 1996. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquir Immune Defic Syndr Hum Retrovirol 12:99–106. - PubMed
-
- Siddappa NB, Watkins JD, Wassermann KJ, Song R, Wang W, Kramer VG, Lakhashe S, Santosuosso M, Poznansky MC, Novembre FJ, Villinger F, Else JG, Montefiori DC, Rasmussen RA, Ruprecht RM. 2010. R5 clade C SHIV strains with tier 1 or 2 neutralization sensitivity: tools to dissect env evolution and to develop AIDS vaccines in primate models. PLoS One 5:e11689. doi:10.1371/journal.pone.0011689. - DOI - PMC - PubMed
-
- Aidoo M, Otten RA, Rodriguez V, Sariol CA, Martinez M, Kraiselburd E, Robinson H, Folks T, Butera S, Ellenberger D. 2007. Absence of SHIV infection in gut and lymph node tissues in rhesus monkeys after repeated rectal challenges following HIV-1 DNA/MVA immunizations. Vaccine 25:6474–6481. doi:10.1016/j.vaccine.2007.06.014. - DOI - PubMed
-
- Li C, Shen Z, Li X, Bai J, Zeng L, Tian M, Song YJ, Du S, Ren D, Liu C, Zhu N, Sun D, Li Y, Jin N. 2012. Protection against SHIV-KB9 infection by combining rDNA and rFPV vaccines based on HIV multiepitope and p24 protein in Chinese rhesus macaques. Clin Dev Immunol 2012:958404. doi:10.1155/2012/958404. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

