Influenza virus M2 protein ion channel activity helps to maintain pandemic 2009 H1N1 virus hemagglutinin fusion competence during transport to the cell surface
- PMID: 25473053
- PMCID: PMC4338904
- DOI: 10.1128/JVI.03253-14
Influenza virus M2 protein ion channel activity helps to maintain pandemic 2009 H1N1 virus hemagglutinin fusion competence during transport to the cell surface
Abstract
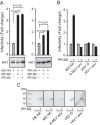
The influenza virus hemagglutinin (HA) envelope protein mediates virus entry by first binding to cell surface receptors and then fusing viral and endosomal membranes during endocytosis. Cleavage of the HA precursor (HA0) into a surface receptor-binding subunit (HA1) and a fusion-inducing transmembrane subunit (HA2) by host cell enzymes primes HA for fusion competence by repositioning the fusion peptide to the newly created N terminus of HA2. We previously reported that the influenza virus M2 protein enhances pandemic 2009 influenza A virus [(H1N1)pdm09] HA-pseudovirus infectivity, but the mechanism was unclear. In this study, using cell-cell fusion and HA-pseudovirus infectivity assays, we found that the ion channel function of M2 was required for enhancement of HA fusion and HA-pseudovirus infectivity. The M2 activity was needed only during HA biosynthesis, and proteolysis experiments indicated that M2 proton channel activity helped to protect (H1N1)pdm09 HA from premature conformational changes as it traversed low-pH compartments during transport to the cell surface. While M2 has previously been shown to protect avian influenza virus HA proteins of the H5 and H7 subtypes that have polybasic cleavage motifs, this study demonstrates that M2 can protect HA proteins from human H1N1 strains that lack a polybasic cleavage motif. This finding suggests that M2 proton channel activity may play a wider role in preserving HA fusion competence among a variety of HA subtypes, including HA proteins from emerging strains that may have reduced HA stability.
Importance: Influenza virus infects cells when the hemagglutinin (HA) surface protein undergoes irreversible pH-induced conformational changes after the virus is taken into the cell by endocytosis. HA fusion competence is primed when host cell enzymes cleave the HA precursor. The proton channel function of influenza virus M2 protein has previously been shown to protect avian influenza virus HA proteins that contain a polybasic cleavage site from pH-induced conformational changes during biosynthesis, but this effect is less well understood for human influenza virus HA proteins that lack polybasic cleavage sites. Using assays that focus on HA entry and fusion, we found that the M2 protein also protects (H1N1)pdm09 influenza A virus HA from premature conformational changes as it transits low-pH compartments during biosynthesis. This work suggests that M2 may play a wider role in preserving HA function in a variety of influenza virus subtypes that infect humans and may be especially important for HA proteins that are less stable.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1648–1689 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

