Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster
- PMID: 25473952
- PMCID: PMC4256086
- DOI: 10.1371/journal.pgen.1004804
Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster
Abstract
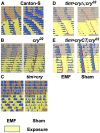
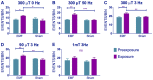
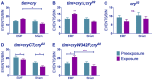
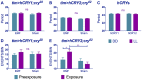
The blue-light sensitive photoreceptor cryptochrome (CRY) may act as a magneto-receptor through formation of radical pairs involving a triad of tryptophans. Previous genetic analyses of behavioral responses of Drosophila to electromagnetic fields using conditioning, circadian and geotaxis assays have lent some support to the radical pair model (RPM). Here, we describe a new method that generates consistent and reliable circadian responses to electromagnetic fields that differ substantially from those already reported. We used the Schuderer apparatus to isolate Drosophila from local environmental variables, and observe extremely low frequency (3 to 50 Hz) field-induced changes in two locomotor phenotypes, circadian period and activity levels. These field-induced phenotypes are CRY- and blue-light dependent, and are correlated with enhanced CRY stability. Mutational analysis of the terminal tryptophan of the triad hypothesised to be indispensable to the electron transfer required by the RPM reveals that this residue is not necessary for field responses. We observe that deletion of the CRY C-terminus dramatically attenuates the EMF-induced period changes, whereas the N-terminus underlies the hyperactivity. Most strikingly, an isolated CRY C-terminus that does not encode the Tryptophan triad nor the FAD binding domain is nevertheless able to mediate a modest EMF-induced period change. Finally, we observe that hCRY2, but not hCRY1, transformants can detect EMFs, suggesting that hCRY2 is blue light-responsive. In contrast, when we examined circadian molecular cycles in wild-type mouse suprachiasmatic nuclei slices under blue light, there was no field effect. Our results are therefore not consistent with the classical Trp triad-mediated RPM and suggest that CRYs act as blue-light/EMF sensors depending on trans-acting factors that are present in particular cellular environments.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kato M (2006) In: Kato M, ed. Electromagnetics in Biology. Tokyo: Springer Japan.
-
- Johnsen S, Lohmann KJ (2008) Magnetoreception in animals. Phys Today 61: 29.
-
- Gould JL (2010) Magnetoreception. Curr Biol 20: R431–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

