Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood
- PMID: 25474103
- PMCID: PMC4256463
- DOI: 10.1371/journal.ppat.1004540
Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood
Abstract
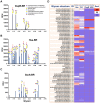
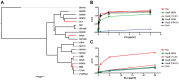
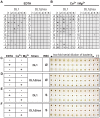
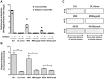
Damaged cardiac valves attract blood-borne bacteria, and infective endocarditis is often caused by viridans group streptococci. While such bacteria use multiple adhesins to maintain their normal oral commensal state, recognition of platelet sialoglycans provides an intermediary for binding to damaged valvular endocardium. We use a customized sialoglycan microarray to explore the varied binding properties of phylogenetically related serine-rich repeat adhesins, the GspB, Hsa, and SrpA homologs from Streptococcus gordonii and Streptococcus sanguinis species, which belong to a highly conserved family of glycoproteins that contribute to virulence for a broad range of Gram-positive pathogens. Binding profiles of recombinant soluble homologs containing novel sialic acid-recognizing Siglec-like domains correlate well with binding of corresponding whole bacteria to arrays. These bacteria show multiple modes of glycan, protein, or divalent cation-dependent binding to synthetic glycoconjugates and isolated glycoproteins in vitro. However, endogenous asialoglycan-recognizing clearance receptors are known to ensure that only fully sialylated glycans dominate in the endovascular system, wherein we find these particular streptococci become primarily dependent on their Siglec-like adhesins for glycan-mediated recognition events. Remarkably, despite an excess of alternate sialoglycan ligands in cellular and soluble blood components, these adhesins selectively target intact bacteria to sialylated ligands on platelets, within human whole blood. These preferred interactions are inhibited by corresponding recombinant soluble adhesins, which also preferentially recognize platelets. Our data indicate that circulating platelets may act as inadvertent Trojan horse carriers of oral streptococci to the site of damaged endocardium, and provide an explanation why it is that among innumerable microbes that gain occasional access to the bloodstream, certain viridans group streptococci have a selective advantage in colonizing damaged cardiac valves and cause infective endocarditis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

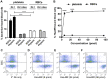
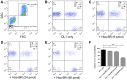
References
-
- Werdan K, Dietz S, Loffler B, Niemann S, Bushnaq H, et al. (2014) Mechanisms of infective endocarditis: pathogen-host interaction and risk states. Nat Rev Cardiol 11: 35–50. - PubMed
-
- Baddour LM, Wilson WR, Bayer AS, Fowler VGJ, Bolger AF, et al. (2005) Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111: e394–e434. - PubMed
-
- Hoen B, Duval X (2013) Clinical practice. Infective endocarditis. N Engl J Med 368: 1425–1433. - PubMed
-
- Duval X, Leport C (2008) Prophylaxis of infective endocarditis: current tendencies, continuing controversies. Lancet Infect Dis 8: 225–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

