Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice
- PMID: 25474590
- PMCID: PMC4256265
- DOI: 10.1371/journal.pgen.1004820
Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice
Abstract
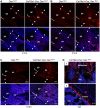
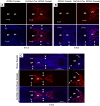
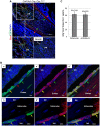
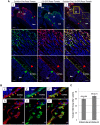
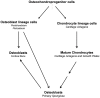
One of the crucial steps in endochondral bone formation is the replacement of a cartilage matrix produced by chondrocytes with bone trabeculae made by osteoblasts. However, the precise sources of osteoblasts responsible for trabecular bone formation have not been fully defined. To investigate whether cells derived from hypertrophic chondrocytes contribute to the osteoblast pool in trabecular bones, we genetically labeled either hypertrophic chondrocytes by Col10a1-Cre or chondrocytes by tamoxifen-induced Agc1-CreERT2 using EGFP, LacZ or Tomato expression. Both Cre drivers were specifically active in chondrocytic cells and not in perichondrium, in periosteum or in any of the osteoblast lineage cells. These in vivo experiments allowed us to follow the fate of cells labeled in Col10a1-Cre or Agc1-CreERT2 -expressing chondrocytes. After the labeling of chondrocytes, both during prenatal development and after birth, abundant labeled non-chondrocytic cells were present in the primary spongiosa. These cells were distributed throughout trabeculae surfaces and later were present in the endosteum, and embedded within the bone matrix. Co-expression studies using osteoblast markers indicated that a proportion of the non-chondrocytic cells derived from chondrocytes labeled by Col10a1-Cre or by Agc1-CreERT2 were functional osteoblasts. Hence, our results show that both chondrocytes prior to initial ossification and growth plate chondrocytes before or after birth have the capacity to undergo transdifferentiation to become osteoblasts. The osteoblasts derived from Col10a1-expressing hypertrophic chondrocytes represent about sixty percent of all mature osteoblasts in endochondral bones of one month old mice. A similar process of chondrocyte to osteoblast transdifferentiation was involved during bone fracture healing in adult mice. Thus, in addition to cells in the periosteum chondrocytes represent a major source of osteoblasts contributing to endochondral bone formation in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Akiyama H, de Crombrugghe B (2009) Transcription control of chondrocyte differentiation. The skeletal system: Cold spring harbor laboratory press. pp. 147–170.
-
- Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M (2008) Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40: 46–62. - PubMed
-
- Gerstenfeld LC, Shapiro FD (1996) Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem 62: 1–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

