Re-response to tolvaptan after furosemide dose reduction in a patient with refractory ascites
- PMID: 25475138
- PMCID: PMC4331598
- DOI: 10.1007/s12328-014-0545-8
Re-response to tolvaptan after furosemide dose reduction in a patient with refractory ascites
Abstract
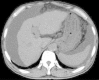
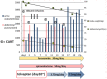
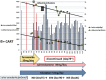
Tolvaptan is a new drug used for treating ascites induced by liver cirrhosis, and it is covered by health insurance in Japan. In the present report, we describe the case of a 74-year-old man with type C liver cirrhosis and refractory ascites. He was receiving furosemide and spironolactone daily, but still required repeat puncture for ascites removal. Administration of tolvaptan (3.75 mg/day) was started in addition to his existing medications, and was subsequently increased to 7.5 mg/day. However, after 2 months, the ascites again exacerbated. Nevertheless, after we discontinued the administration of furosemide, the tolvaptan became effective. This may be because furosemide administration decreases urine osmolality, resulting in a non-response to tolvaptan.
Figures
Similar articles
-
Cases with Refractory Ascites and a Delayed Response to Tolvaptan.Intern Med. 2016;55(22):3273-3277. doi: 10.2169/internalmedicine.55.7035. Epub 2016 Nov 15. Intern Med. 2016. PMID: 27853068 Free PMC article.
-
Clinical Factors Predicting the Effect of Tolvaptan for Refractory Ascites in Patients with Decompensated Liver Cirrhosis.Dig Dis. 2016;34(6):659-664. doi: 10.1159/000448828. Epub 2016 Oct 17. Dig Dis. 2016. PMID: 27750234
-
Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients.World J Gastroenterol. 2014 Aug 28;20(32):11400-5. doi: 10.3748/wjg.v20.i32.11400. World J Gastroenterol. 2014. PMID: 25170228 Free PMC article.
-
[Management of ascites in cirrhotic patients].Nihon Shokakibyo Gakkai Zasshi. 2017;114(1):27-34. doi: 10.11405/nisshoshi.114.27. Nihon Shokakibyo Gakkai Zasshi. 2017. PMID: 28070092 Review. Japanese. No abstract available.
-
Tolvaptan for the treatment of liver cirrhosis oedema.Expert Rev Gastroenterol Hepatol. 2014 Jul;8(5):461-70. doi: 10.1586/17474124.2014.903797. Epub 2014 Mar 29. Expert Rev Gastroenterol Hepatol. 2014. PMID: 24678622 Review.
Cited by
-
Cisterna chyli as an optimal marker of tolvaptan response in severe cirrhotic ascites.Sci Rep. 2022 May 17;12(1):8124. doi: 10.1038/s41598-022-11889-z. Sci Rep. 2022. PMID: 35581243 Free PMC article.
References
-
- Yamamura Y, Nakamura S, Itoh S, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–867. - PubMed
-
- Matsuzaki M, Hori M, Izumi T, et al. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study) Cardiovasc Drugs Ther. 2011;25:S33–S45. doi: 10.1007/s10557-011-6304-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

