Demonstration of extracellular peptidylarginine deiminase (PAD) activity in synovial fluid of patients with rheumatoid arthritis using a novel assay for citrullination of fibrinogen
- PMID: 25475141
- PMCID: PMC4298085
- DOI: 10.1186/s13075-014-0498-9
Demonstration of extracellular peptidylarginine deiminase (PAD) activity in synovial fluid of patients with rheumatoid arthritis using a novel assay for citrullination of fibrinogen
Abstract
Introduction: Members of the peptidylarginine deiminase (PAD) family catalyse the posttranslational conversion of peptidylarginine to peptidylcitrulline. Citrullination of proteins is well described in rheumatoid arthritis (RA), and hypercitrullination of proteins may be related to inflammation in general. PAD activity has been demonstrated in various cell lysates, but so far not in synovial fluid. We aimed to develop an assay for detection of PAD activity, if any, in synovial fluid from RA patients.
Methods: An enzyme-linked immunosorbent assay using human fibrinogen as the immobilized substrate for citrullination and anti-citrullinated fibrinogen antibody as the detecting agent were used for measurement of PAD activity in synovial fluid samples from five RA patients. The concentrations of PAD2 and calcium were also determined.
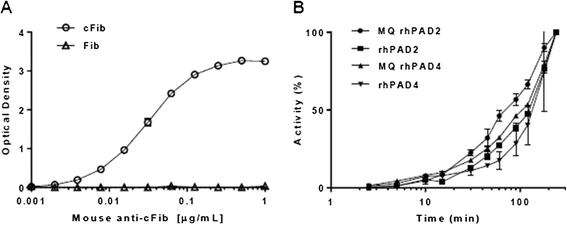
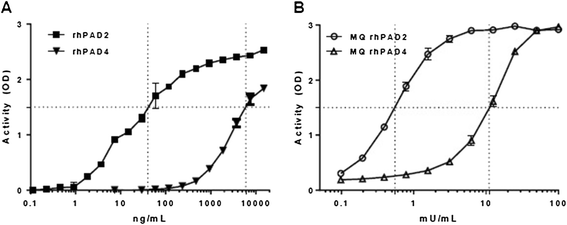
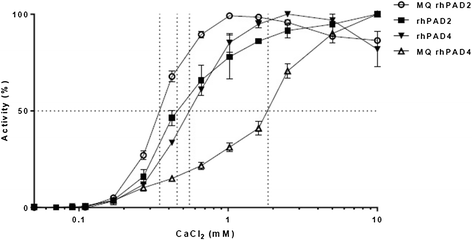
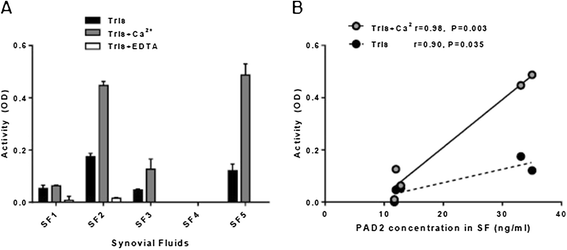
Results: Approximately 150 times lower levels of recombinant human PAD2 (rhPAD2) than of rhPAD4 were required for citrullination of fibrinogen. PAD activity was detected in four of five synovial fluid samples from RA patients and correlated with PAD2 concentrations in the samples (r = 0.98, P = 0.003). The calcium requirement for half-maximal activities of PAD2 and PAD4 were found in a range from 0.35 to 1.85 mM, and synovial fluid was found to contain sufficient calcium levels for the citrullination process to occur.
Conclusions: We present an assay with high specificity for PAD2 activity and show that citrullination of fibrinogen can occur in cell-free synovial fluid from RA patients.
Figures
Similar articles
-
Reduced glutathione as a physiological co-activator in the activation of peptidylarginine deiminase.Arthritis Res Ther. 2016 May 5;18(1):102. doi: 10.1186/s13075-016-1000-7. Arthritis Res Ther. 2016. PMID: 27149996 Free PMC article.
-
Citrullination of synovial proteins in murine models of rheumatoid arthritis.Arthritis Rheum. 2003 Sep;48(9):2489-500. doi: 10.1002/art.11229. Arthritis Rheum. 2003. PMID: 13130468
-
High-Titer Rheumatoid Arthritis Antibodies Preferentially Bind Fibrinogen Citrullinated by Peptidylarginine Deiminase 4.Arthritis Rheumatol. 2017 May;69(5):986-995. doi: 10.1002/art.40035. Arthritis Rheumatol. 2017. PMID: 28029744 Free PMC article.
-
Peptidylarginine deiminase (PAD): A promising target for chronic diseases treatment.Int J Biol Macromol. 2024 Oct;278(Pt 3):134576. doi: 10.1016/j.ijbiomac.2024.134576. Epub 2024 Aug 9. Int J Biol Macromol. 2024. PMID: 39127273 Review.
-
Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination.Mol Cell Proteomics. 2014 Feb;13(2):388-96. doi: 10.1074/mcp.R113.033746. Epub 2013 Dec 2. Mol Cell Proteomics. 2014. PMID: 24298040 Free PMC article. Review.
Cited by
-
Inflammatory but not apoptotic death of granulocytes citrullinates fibrinogen.Arthritis Res Ther. 2015 Dec 17;17:369. doi: 10.1186/s13075-015-0890-0. Arthritis Res Ther. 2015. PMID: 26684871 Free PMC article.
-
Cigarette Smoke Induces Immune Responses to Vimentin in both, Arthritis-Susceptible and -Resistant Humanized Mice.PLoS One. 2016 Sep 7;11(9):e0162341. doi: 10.1371/journal.pone.0162341. eCollection 2016. PLoS One. 2016. PMID: 27602574 Free PMC article.
-
Reactive oxygen species inhibit catalytic activity of peptidylarginine deiminase.J Enzyme Inhib Med Chem. 2017 Dec;32(1):1203-1208. doi: 10.1080/14756366.2017.1368505. J Enzyme Inhib Med Chem. 2017. PMID: 28933232 Free PMC article.
-
Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis.Nat Commun. 2020 Aug 25;11(1):4243. doi: 10.1038/s41467-020-17749-6. Nat Commun. 2020. PMID: 32843625 Free PMC article.
-
Autoantibodies to Peptidylarginine Deiminase 2 Are Associated With Less Severe Disease in Rheumatoid Arthritis.Front Immunol. 2018 Nov 20;9:2696. doi: 10.3389/fimmu.2018.02696. eCollection 2018. Front Immunol. 2018. PMID: 30515171 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

