Integrative medicine for subacute stroke rehabilitation: a study protocol for a multicentre, randomised, controlled trial
- PMID: 25475247
- PMCID: PMC4256536
- DOI: 10.1136/bmjopen-2014-007080
Integrative medicine for subacute stroke rehabilitation: a study protocol for a multicentre, randomised, controlled trial
Abstract
Introduction: Many patients with stroke receive integrative medicine in China, which includes the basic treatment of Western medicine and routine rehabilitation, in conjunction with acupuncture and Chinese medicine. The question of whether integrative medicine is efficacious for stroke rehabilitation is still controversial and very little research currently exists on the integrated approach for this condition. Consequently, we will conduct a multicentre, randomised, controlled, assessor-blinded clinical trial to assess the effectiveness of integrative medicine on stroke rehabilitation.
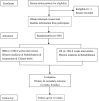
Methods and analysis: 360 participants recruited from three large Chinese medical hospitals in Zhejiang Province will be randomly divided into the integrative medicine rehabilitation (IMR) group and the conventional rehabilitation (CR) group in a 1:1 ratio. Participants in the IMR group will receive acupuncture and Chinese herbs in addition to basic Western medicine and rehabilitation treatment. The CR group will not receive acupuncture and Chinese herbal medicine. The assessment data will be collected at baseline, 4 and 8 weeks postrandomisation, and then at 12 weeks' follow-up. The primary outcome is measured by the Modified Barthel Index. The secondary outcomes are the National Institutes of Health Stroke Scale (NIHSS), Fugl-Meyer Assessment, the mini-mental state examination and Montreal Cognitive, Hamilton's Depression Scale and Self-Rating Depression Scale, and the incidence of adverse events.
Ethics and dissemination: Ethical approval was obtained from ethics committees of three hospitals. The results will be disseminated in a peer-reviewed journal and presented at international congresses. The results will also be disseminated to patients by telephone, during follow-up calls inquiring on patient's post-study health status.
Trial registration number: Chinese Clinical Trial Register: ChiCTR-TRC-12001972, http://www.chictr.org/en/proj/show.aspx?proj=2561.
Keywords: COMPLEMENTARY MEDICINE; STROKE MEDICINE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Bonita R, Mendis S, Truelsen T et al. . The global stroke initiative. Lancet Neurol 2004;3:391–3. - PubMed
-
- Wang L, Kong L, Wu F et al. . Preventing chronic diseases in China. Lancet 2005;366:1821–4. - PubMed
-
- Liu M, Wu B, Wang WZ et al. . Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007;6:456–64. - PubMed
-
- Liu L, Wang D, Wong KS et al. . Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011;42:3651–4. - PubMed
-
- Zhao D, Liu J, Wang W et al. . Epidemiological transition of stroke in China twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke 2008;39:1668–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials