Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units
- PMID: 25475356
- PMCID: PMC4346713
- DOI: 10.1152/jn.00777.2014
Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units
Abstract
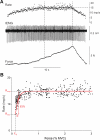
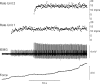
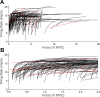
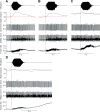
During voluntary contraction, firing rates of individual motor units (MUs) increase modestly over a narrow force range beyond which little additional increase in firing rate is seen. Such saturation of MU discharge may be a consequence of extrinsic factors that limit net synaptic excitation acting on motor neurons (MNs) or may be due to intrinsic properties of the MNs. Two sets of experiments involving recording of human biceps brachii MUs were carried out to evaluate saturation. In the first set, the extent of saturation was quantified for 136 low-threshold MUs during isometric ramp contractions. Firing rate-force data were best fit by a saturating function for 90% of MUs recorded with a maximum rate of 14.8 ± 2.0 impulses/s. In the second set of experiments, to distinguish extrinsic from intrinsic factors underlying saturation, we artificially augmented descending excitatory drive to biceps MNs by activation of muscle spindle afferents through tendon vibration. We examined the change in firing rate caused by tendon vibration in 96 MUs that were voluntarily activated at rates below and at saturation. Vibration had little effect on the discharge of MUs that were firing at saturation frequencies but strongly increased firing rates of the same units when active at lower frequencies. These results indicate that saturation is likely caused by intrinsic mechanisms that prevent further increases in firing rate in the presence of increasing synaptic excitation. Possible intrinsic cellular mechanisms that limit firing rates of motor units during voluntary effort are discussed.
Keywords: firing rate; force; motor neuron; motor unit; saturation.
Copyright © 2015 the American Physiological Society.
Figures


Similar articles
-
Inhibition linearizes firing rate responses in human motor units: implications for the role of persistent inward currents.J Physiol. 2017 Jan 1;595(1):179-191. doi: 10.1113/JP272823. Epub 2016 Sep 20. J Physiol. 2017. PMID: 27470946 Free PMC article.
-
Prolonged firing in motor units: evidence of plateau potentials in human motoneurons?J Neurophysiol. 1997 Dec;78(6):3061-8. doi: 10.1152/jn.1997.78.6.3061. J Neurophysiol. 1997. PMID: 9405525 Clinical Trial.
-
Ia Afferent input alters the recruitment thresholds and firing rates of single human motor units.Exp Brain Res. 2003 Jun;150(4):449-57. doi: 10.1007/s00221-003-1463-z. Epub 2003 May 9. Exp Brain Res. 2003. PMID: 12739088
-
Firing rate trajectories of human motor units during isometric ramp contractions to 10, 25 and 50% of maximal voluntary contraction.Neurosci Lett. 2021 Sep 25;762:136118. doi: 10.1016/j.neulet.2021.136118. Epub 2021 Jul 17. Neurosci Lett. 2021. PMID: 34280505
-
Influence of exercise and training on motor unit activation.Exerc Sport Sci Rev. 1987;15:95-151. Exerc Sport Sci Rev. 1987. PMID: 3297731 Review.
Cited by
-
Force variability is mostly not motor noise: Theoretical implications for motor control.PLoS Comput Biol. 2021 Mar 8;17(3):e1008707. doi: 10.1371/journal.pcbi.1008707. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33684099 Free PMC article.
-
The Cellular Basis for the Generation of Firing Patterns in Human Motor Units.Adv Neurobiol. 2022;28:233-258. doi: 10.1007/978-3-031-07167-6_10. Adv Neurobiol. 2022. PMID: 36066828
-
A modeling study of spinal motoneuron recruitment regulated by ionic channels during fictive locomotion.J Comput Neurosci. 2020 Nov;48(4):409-428. doi: 10.1007/s10827-020-00763-4. Epub 2020 Sep 8. J Comput Neurosci. 2020. PMID: 32895895
-
Decoding firings of a large population of human motor units from high-density surface electromyogram in response to transcranial magnetic stimulation.J Physiol. 2023 May;601(10):1719-1744. doi: 10.1113/JP284043. Epub 2023 Apr 5. J Physiol. 2023. PMID: 36946417 Free PMC article.
-
Amplitude cancellation influences the association between frequency components in the neural drive to muscle and the rectified EMG signal.PLoS Comput Biol. 2019 May 3;15(5):e1006985. doi: 10.1371/journal.pcbi.1006985. eCollection 2019 May. PLoS Comput Biol. 2019. PMID: 31050667 Free PMC article.
References
-
- Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97: 933–936, 2007. - PubMed
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

