Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure
- PMID: 25475392
- PMCID: PMC4736545
- DOI: 10.3109/17435390.2014.984251
Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure
Abstract
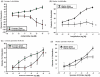
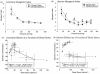
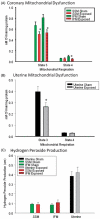
Due to the ongoing evolution of nanotechnology, there is a growing need to assess the toxicological outcomes in under-studied populations in order to properly consider the potential of engineered nanomaterials (ENM) and fully enhance their safety. Recently, we and others have explored the vascular consequences associated with gestational nanomaterial exposure, reporting microvascular dysfunction within the uterine circulation of pregnant dams and the tail artery of fetal pups. It has been proposed (via work derived by the Barker Hypothesis) that mitochondrial dysfunction and subsequent oxidative stress mechanisms as a possible link between a hostile gestational environment and adult disease. Therefore, in this study, we exposed pregnant Sprague-Dawley rats to nanosized titanium dioxide aerosols after implantation (gestational day 6). Pups were delivered, and the progeny grew into adulthood. Microvascular reactivity, mitochondrial respiration and hydrogen peroxide production of the coronary and uterine circulations of the female offspring were evaluated. While there were no significant differences within the maternal or litter characteristics, endothelium-dependent dilation and active mechanotransduction in both coronary and uterine arterioles were significantly impaired. In addition, there was a significant reduction in maximal mitochondrial respiration (state 3) in the left ventricle and uterus. These studies demonstrate microvascular dysfunction and coincide with mitochondrial inefficiencies in both the cardiac and uterine tissues, which may represent initial evidence that prenatal ENM exposure produces microvascular impairments that persist throughout multiple developmental stages.
Keywords: Barker Hypothesis; engineered nanomaterials; nanotoxicology; pregnancy; prenatal.
Figures
References
-
- Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
