Semaphorin3a promotes advanced diabetic nephropathy
- PMID: 25475434
- PMCID: PMC4407856
- DOI: 10.2337/db14-0719
Semaphorin3a promotes advanced diabetic nephropathy
Abstract
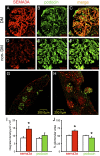
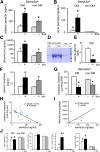
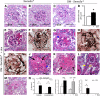
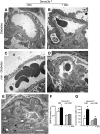
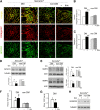
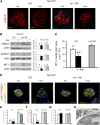
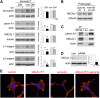
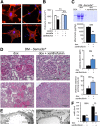
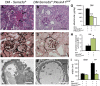
The onset of diabetic nephropathy (DN) is highlighted by glomerular filtration barrier abnormalities. Identifying pathogenic factors and targetable pathways driving DN is crucial to developing novel therapies and improving the disease outcome. Semaphorin3a (sema3a) is a guidance protein secreted by podocytes. Excess sema3a disrupts the glomerular filtration barrier. Here, using immunohistochemistry, we show increased podocyte SEMA3A in renal biopsies from patients with advanced DN. Using inducible, podocyte-specific Sema3a gain-of-function (Sema3a(+)) mice made diabetic with streptozotocin, we demonstrate that sema3a is pathogenic in DN. Diabetic Sema3a(+) mice develop massive proteinuria, renal insufficiency, and extensive nodular glomerulosclerosis, mimicking advanced DN in humans. In diabetic mice, Sema3a(+) exacerbates laminin and collagen IV accumulation in Kimmelstiel-Wilson-like glomerular nodules and causes diffuse podocyte foot process effacement and F-actin collapse via nephrin, αvβ3 integrin, and MICAL1 interactions with plexinA1. MICAL1 knockdown and sema3a inhibition render podocytes not susceptible to sema3a-induced shape changes, indicating that MICAL1 mediates sema3a-induced podocyte F-actin collapse. Moreover, sema3a binding inhibition or podocyte-specific plexinA1 deletion markedly ameliorates albuminuria and abrogates renal insufficiency and the diabetic nodular glomerulosclerosis phenotype of diabetic Sema3a(+) mice. Collectively, these findings indicate that excess sema3a promotes severe diabetic nephropathy and identifies novel potential therapeutic targets for DN.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
Similar articles
-
Excess podocyte semaphorin-3A leads to glomerular disease involving plexinA1-nephrin interaction.Am J Pathol. 2013 Oct;183(4):1156-1168. doi: 10.1016/j.ajpath.2013.06.022. Epub 2013 Aug 14. Am J Pathol. 2013. PMID: 23954273 Free PMC article.
-
Podocyte-specific VEGF-a gain of function induces nodular glomerulosclerosis in eNOS null mice.J Am Soc Nephrol. 2014 Aug;25(8):1814-24. doi: 10.1681/ASN.2013070752. Epub 2014 Feb 27. J Am Soc Nephrol. 2014. PMID: 24578128 Free PMC article.
-
Podocyte vascular endothelial growth factor (Vegf₁₆₄) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes.Diabetologia. 2011 May;54(5):1227-41. doi: 10.1007/s00125-010-2034-z. Epub 2011 Feb 12. Diabetologia. 2011. PMID: 21318407 Free PMC article.
-
Semaphorin3a signaling, podocyte shape, and glomerular disease.Pediatr Nephrol. 2014 Apr;29(4):751-5. doi: 10.1007/s00467-013-2743-x. Epub 2014 Jan 26. Pediatr Nephrol. 2014. PMID: 24464477 Free PMC article. Review.
-
Glomerular podocytes in diabetic renal disease.Adv Clin Exp Med. 2019 Dec;28(12):1711-1715. doi: 10.17219/acem/104534. Adv Clin Exp Med. 2019. PMID: 31851794 Review.
Cited by
-
Decreased Serum microRNA-21, microRNA-25, microRNA-146a, and microRNA-181a in Autoimmune Diabetes: Potential Biomarkers for Diagnosis and Possible Involvement in Pathogenesis.Int J Endocrinol. 2019 Sep 9;2019:8406438. doi: 10.1155/2019/8406438. eCollection 2019. Int J Endocrinol. 2019. PMID: 31582977 Free PMC article.
-
Axon guidance molecules in immunometabolic diseases.Inflamm Regen. 2022 Jan 19;42(1):5. doi: 10.1186/s41232-021-00189-0. Inflamm Regen. 2022. PMID: 35045890 Free PMC article. Review.
-
Expression analysis and possible functional roles of semaphorin/plexin/CRMP families in mouse pancreatic islets.Sci Rep. 2025 Mar 27;15(1):10546. doi: 10.1038/s41598-025-95300-7. Sci Rep. 2025. PMID: 40148522 Free PMC article.
-
MICAL1 Mediates TGF-β1-Induced Epithelial-to-Mesenchymal Transition and Metastasis of Hepatocellular Carcinoma by Activating Smad2/3.Cell Biochem Biophys. 2025 Jun;83(2):2589-2606. doi: 10.1007/s12013-025-01668-8. Epub 2025 Feb 15. Cell Biochem Biophys. 2025. PMID: 39954154
-
LncRNA TCF7 contributes to high glucose-induced damage in human podocytes by up-regulating SEMA3A via sponging miR-16-5p.J Diabetes Investig. 2023 Feb;14(2):193-204. doi: 10.1111/jdi.13904. Epub 2022 Dec 29. J Diabetes Investig. 2023. PMID: 36583231 Free PMC article.
References
-
- Fineberg D, Jandeleit-Dahm KA, Cooper ME. Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol 2013;9:713–723 - PubMed
-
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013;93:137–188 - PubMed
-
- Breyer MD. Drug discovery for diabetic nephropathy: trying the leap from mouse to man. Semin Nephrol 2012;32:445–451 - PubMed
-
- Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983;32(Suppl. 2):64–78 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

