Protein Ingestion Induces Muscle Insulin Resistance Independent of Leucine-Mediated mTOR Activation
- PMID: 25475435
- PMCID: PMC4407849
- DOI: 10.2337/db14-1279
Protein Ingestion Induces Muscle Insulin Resistance Independent of Leucine-Mediated mTOR Activation
Abstract
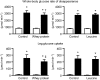
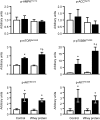
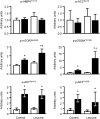
Increased plasma branched-chain amino acid concentrations are associated with insulin resistance, and intravenous amino acid infusion blunts insulin-mediated glucose disposal. We tested the hypothesis that protein ingestion impairs insulin-mediated glucose disposal by leucine-mediated mTOR signaling, which can inhibit AKT. We measured glucose disposal and muscle p-mTOR(Ser2448), p-AKT(Ser473), and p-AKT(Thr308) in 22 women during a hyperinsulinemic-euglycemic clamp procedure with and without concomitant ingestion of whey protein (0.6 g/kg fat-free mass; n = 11) or leucine that matched the amount given with whey protein (n = 11). Both whey protein and leucine ingestion raised plasma leucine concentration by approximately twofold and muscle p-mTOR(Ser2448) by ∼30% above the values observed in the control (no amino acid ingestion) studies; p-AKT(Ser473) and p-AKT(Thr308) were not affected by whey protein or leucine ingestion. Whey protein ingestion decreased insulin-mediated glucose disposal (median 38.8 [quartiles 30.8, 61.8] vs. 51.9 [41.0, 77.3] µmol glucose/µU insulin · mL(-1) · min(-1); P < 0.01), whereas ingestion of leucine did not (52.3 [43.3, 65.4] vs. 52.3 [43.9, 73.2]). These results indicate that 1) protein ingestion causes insulin resistance and could be an important regulator of postprandial glucose homeostasis and 2) the insulin-desensitizing effect of protein ingestion is not due to inhibition of AKT by leucine-mediated mTOR signaling.
Trial registration: ClinicalTrials.gov NCT01538836 NCT01757340.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
Comment in
-
Comment on Smith et al. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 2015;64:1555-1563.Diabetes. 2015 Jun;64(6):e10. doi: 10.2337/db15-0149. Diabetes. 2015. PMID: 25999539 No abstract available.
-
Response to Comment on Smith et al. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 2015;64:1555-1563.Diabetes. 2015 Jun;64(6):e11. doi: 10.2337/db15-0225. Diabetes. 2015. PMID: 25999540 Free PMC article. No abstract available.
Similar articles
-
Comment on Smith et al. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 2015;64:1555-1563.Diabetes. 2015 Jun;64(6):e10. doi: 10.2337/db15-0149. Diabetes. 2015. PMID: 25999539 No abstract available.
-
Response to Comment on Smith et al. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 2015;64:1555-1563.Diabetes. 2015 Jun;64(6):e11. doi: 10.2337/db15-0225. Diabetes. 2015. PMID: 25999540 Free PMC article. No abstract available.
-
The muscle anabolic effect of protein ingestion during a hyperinsulinaemic euglycaemic clamp in middle-aged women is not caused by leucine alone.J Physiol. 2018 Oct;596(19):4681-4692. doi: 10.1113/JP276504. Epub 2018 Aug 29. J Physiol. 2018. PMID: 30054913 Free PMC article.
-
Modulation of insulin action by dietary proteins and amino acids: role of the mammalian target of rapamycin nutrient sensing pathway.Curr Opin Clin Nutr Metab Care. 2005 Jul;8(4):457-62. doi: 10.1097/01.mco.0000172589.55434.03. Curr Opin Clin Nutr Metab Care. 2005. PMID: 15930974 Review.
-
High dietary protein intake, reducing or eliciting insulin resistance?Eur J Clin Nutr. 2014 Sep;68(9):973-9. doi: 10.1038/ejcn.2014.123. Epub 2014 Jul 2. Eur J Clin Nutr. 2014. PMID: 24986822 Review.
Cited by
-
Effects of Branched-Chain Amino Acids on Skeletal Muscle, Glycemic Control, and Neuropsychological Performance in Elderly Persons with Type 2 Diabetes Mellitus: An Exploratory Randomized Controlled Trial.Nutrients. 2022 Sep 21;14(19):3917. doi: 10.3390/nu14193917. Nutrients. 2022. PMID: 36235570 Free PMC article. Clinical Trial.
-
A Short-Term Feeding of Dietary Casein Increases Abundance of Lactococcus lactis and Upregulates Gene Expression Involving Obesity Prevention in Cecum of Young Rats Compared With Dietary Chicken Protein.Front Microbiol. 2019 Oct 25;10:2411. doi: 10.3389/fmicb.2019.02411. eCollection 2019. Front Microbiol. 2019. PMID: 31708891 Free PMC article.
-
Total energy intake according to the level of skeletal muscle mass in Korean adults aged 30 years and older: an analysis of the Korean National Health and Nutrition Examination Surveys (KNHANES) 2008-2011.Nutr Res Pract. 2018 Jun;12(3):222-232. doi: 10.4162/nrp.2018.12.3.222. Epub 2018 May 23. Nutr Res Pract. 2018. PMID: 29854328 Free PMC article.
-
Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity.Nutrients. 2022 Dec 23;15(1):68. doi: 10.3390/nu15010068. Nutrients. 2022. PMID: 36615726 Free PMC article. Review.
-
SGLT2 inhibitor versus carbohydrate-restricted isocaloric diet: reprogramming substrate oxidation in type 2 diabetes.Diabetol Metab Syndr. 2023 Feb 19;15(1):25. doi: 10.1186/s13098-023-00990-6. Diabetol Metab Syndr. 2023. PMID: 36804863 Free PMC article.
References
-
- Prager R, Wallace P, Olefsky JM. Hyperinsulinemia does not compensate for peripheral insulin resistance in obesity. Diabetes 1987;36:327–334 - PubMed
-
- Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014;56:369–381 - PubMed
-
- Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012;43:171–181 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

