Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross-sectional survey
- PMID: 25475751
- PMCID: PMC4594724
- DOI: 10.1111/bcp.12567
Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross-sectional survey
Abstract
Aims: Fatal adverse drug reactions (ADRs) are important causes of death, but data from resource-limited settings are scarce. We determined the proportion of deaths in South African medical inpatients attributable to ADRs, and their preventability, stratified by human immunodeficiency virus (HIV) status.
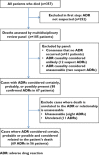
Methods: We reviewed the folders of all patients who died over a 30 day period in the medical wards of four hospitals. We identified ADR-related deaths (deaths where an ADR was 'possible', 'probable' or 'certain' using WHO-UMC criteria and where the ADR contributed to death). We determined preventability according to previously published criteria.
Results: ADRs contributed to the death of 2.9% of medical admissions and 56 of 357 deaths (16%) were ADR-related. Tenofovir, rifampicin and co-trimoxazole were the most commonly implicated drugs. 43% of ADRs were considered preventable. The following factors were independently associated with ADR-related death: HIV-infected patients on antiretroviral therapy (adjusted odds ratio (aOR) 4.4, 95% confidence interval (CI) 1.6, 12), exposure to more than seven drugs (aOR 2.5, 95% CI 1.3, 4.8) and increasing comorbidity score (aOR 1.3, 95% CI 1.1, 1.7).
Conclusions: In our setting, where HIV and tuberculosis are highly prevalent, fatal in-hospital ADRs were more common than reported in high income settings. Most deaths were attributed to drugs used in managing HIV and tuberculosis. A large proportion of the ADRs were preventable, highlighting the need to strengthen systems for health care worker training and support.
Keywords: South Africa; adverse drug reaction; anti-tuberculosis therapy; antiretroviral therapy; in-hospital death; preventability.
© 2014 The British Pharmacological Society.
Figures
References
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Buajordet I, Ebbesen J, Erikssen J, Brørs O, Hilberg T. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250:327–341. - PubMed
-
- Ebbesen J, Buajordet I, Erikssen J, Brørs O, Hilberg T, Svaar H, Sandvik L. Drug-related deaths in a department of internal medicine. Arch Intern Med. 2001;161:2317–2323. - PubMed
-
- Juntti-Patinen L, Neuvonen PJ. Drug-related deaths in a university central hospital. Eur J Clin Pharmacol. 2002;58:479–482. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials