Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials
- PMID: 25475878
- PMCID: PMC4516407
- DOI: 10.1177/1740774514558307
Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials
Abstract
Background: Ethical evaluation of risk-benefit in clinical trials is premised on the achievability of resolving research questions motivating an investigation.
Objective: To determine the fraction and number of patients enrolled in trials that were at risk of not meaningfully addressing their primary research objective due to unsuccessful patient accrual.
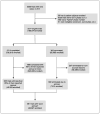
Methods: We used the National Library of Medicine clinical trial registry to capture all initiated phases 2 and 3 intervention clinical trials that were registered as closed in 2011. We then determined the number that had been terminated due to unsuccessful accrual and the number that had closed after less than 85% of the target number of human subjects had been enrolled. Five factors were tested for association with unsuccessful accrual.
Results: Of 2579 eligible trials, 481 (19%) either terminated for failed accrual or completed with less than 85% expected enrolment, seriously compromising their statistical power. Factors associated with unsuccessful accrual included greater number of eligibility criteria (p = 0.013), non-industry funding (25% vs 16%, p < 0.0001), earlier trial phase (23% vs 16%, p < 0.0001), fewer number of research sites at trial completion (p < 0.0001) and at registration (p < 0.0001), and an active (non-placebo) comparator (23% vs 16%, p < 0.001).
Conclusion: A total of 48,027 patients had enrolled in trials closed in 2011 who were unable to answer the primary research question meaningfully. Ethics bodies, investigators, and data monitoring committees should carefully scrutinize trial design, recruitment plans, and feasibility of achieving accrual targets when designing and reviewing trials, monitor accrual once initiated, and take corrective action when accrual is lagging.
Keywords: Medical ethics; clinical trials; recruitment; research ethics; trial accrual.
© The Author(s) 2014.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- World Medical Association. Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 2011 - PubMed
-
- U.S. Department of Health & Human Services. Code of Federal Regulations: Title 45 - Public Welfare - Part 46 - Protection of Human Subjects, 45 CFR 46. 2009 - PubMed
-
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research; Department of Health Education and Welfare, editor. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. 1979. - PubMed
-
- Cheng SK, Dietrich MS, Dilts DM. A sense of urgency: Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:5557–63. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources