Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells
- PMID: 25475897
- PMCID: PMC4303198
- DOI: 10.1186/s13058-014-0489-4
Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells
Abstract
Introduction: Luminal, estrogen receptor-positive (ER(+)) breast cancers can metastasize but lie dormant for years before recurrences prove lethal. Understanding the roles of estrogen (E) or progestin (P) in development of luminal metastases or in arousal from dormancy is hindered by few preclinical models. We have developed such models.
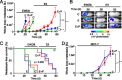
Methods: Immunocompromised, ovariectomized (ovx'd) mice were intracardiac-injected with luminal or basal human breast cancer cells. Four lines were tested: luminal ER(+)PR(+) cytokeratin 5-negative (CK5(-)) E3 and MCF-7 cells, basal ER(-)PR(-)CK5(+) estrogen withdrawn-line 8 (EWD8) cells, and basal ER(-)PR(-)CK5(-) MDA-MB-231 cells. Development of micrometastases or macrometastases was quantified in ovx'd mice and in mice supplemented with E or P or both. Metastatic deposits were analyzed by immunohistochemistry for luminal, basal, and proliferation markers.
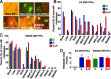
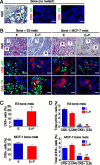
Results: ER(-)PR(-) cells generated macrometastases in multiple organs in the absence or presence of hormones. By contrast, ovx'd mice injected with ER(+)PR(+) cells appeared to be metastases-free until they were supplemented with E or E+P. Furthermore, unlike parental ER(+)PR(+)CK5(-) cells, luminal metastases were heterogeneous, containing a significant (6% to 30%) proportion of non-proliferative ER(-)PR(-)CK5(+) cells that would be chemotherapy-resistant. Additionally, because these cells lack receptors, they would also be endocrine therapy-resistant. With regard to ovx'd control mice injected with ER(+)PR(+) cells that appeared to be metastases-free, systematic pathologic analysis of organs showed that some harbor a reservoir of dormant micrometastases that are ER(+) but PR(-). Such cells may also be endocrine therapy- and chemotherapy-resistant. Their emergence as macrometastases can be triggered by E or E+P restoration.
Conclusions: We conclude that hormones promote development of multi-organ macrometastases in luminal disease. The metastases display a disturbing heterogeneity, containing newly emergent ER(-)PR(-) subpopulations that would be resistant to endocrine therapy and chemotherapy. Similar cells are found in luminal metastases of patients. Furthermore, lack of hormones is not protective. While no overt metastases form in ovx'd mice, luminal tumor cells can seed distant organs, where they remain dormant as micrometastases and sheltered from therapies but arousable by hormone repletion. This has implications for breast cancer survivors or women with occult disease who are prescribed hormones for contraception or replacement purposes.
Figures

References
-
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. - DOI - PMC - PubMed
-
- Demicheli R, Biganzoli E, Ardoino I, Boracchi P, Coradini D, Greco M, Moliterni A, Zambetti M, Valagussa P, Gukas ID, Bonadonna G. Recurrence and mortality dynamics for breast cancer patients undergoing mastectomy according to estrogen receptor status: different mortality but similar recurrence. Cancer Sci. 2010;101:826–830. doi: 10.1111/j.1349-7006.2009.01472.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

