Genome-wide screen for modifiers of Na (+) /K (+) ATPase alleles identifies critical genetic loci
- PMID: 25476251
- PMCID: PMC4302446
- DOI: 10.1186/s13041-014-0089-3
Genome-wide screen for modifiers of Na (+) /K (+) ATPase alleles identifies critical genetic loci
Abstract
Background: Mutations affecting the Na (+) / K (+) ATPase (a.k.a. the sodium-potassium pump) genes cause conditional locomotor phenotypes in flies and three distinct complex neurological diseases in humans. More than 50 mutations have been identified affecting the human ATP1A2 and ATP1A3 genes that are known to cause rapid-onset Dystonia Parkinsonism, familial hemiplegic migraine, alternating hemiplegia of childhood, and variants of familial hemiplegic migraine with neurological complications including seizures and various mood disorders. In flies, mutations affecting the ATPalpha gene have dramatic phenotypes including altered longevity, neural dysfunction, neurodegeneration, myodegeneration, and striking locomotor impairment. Locomotor defects can manifest as conditional bang-sensitive (BS) or temperature-sensitive (TS) paralysis: phenotypes well-suited for genetic screening.
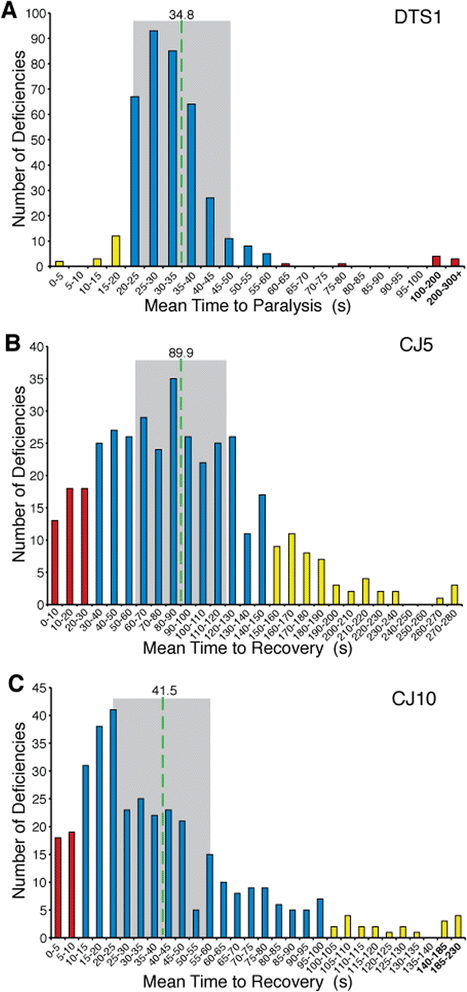
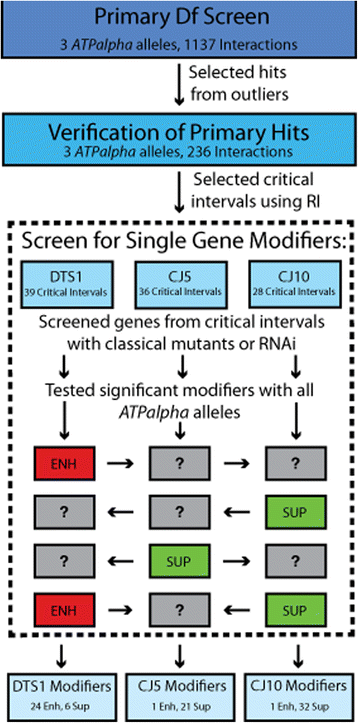
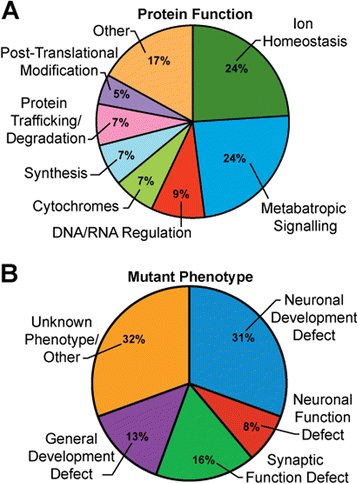
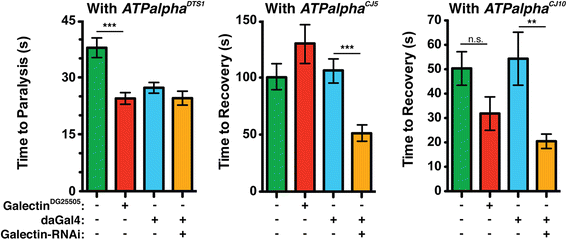
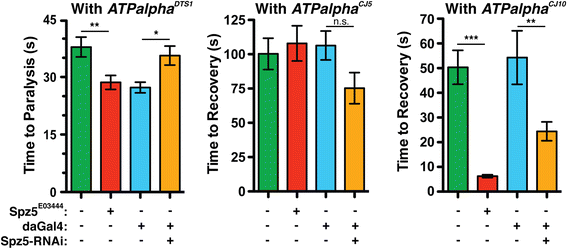
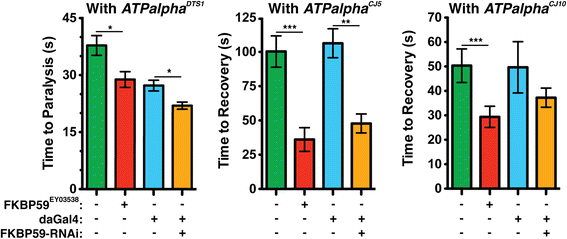
Results: We performed a genome-wide deficiency screen using three distinct missense alleles of ATPalpha and conditional locomotor function assays to identify novel modifier loci. A secondary screen confirmed allele-specificity of the interactions and many of the interactions were mapped to single genes and subsequently validated. We successfully identified 64 modifier loci and used classical mutations and RNAi to confirm 50 single gene interactions. The genes identified include those with known function, several with unknown function or that were otherwise uncharacterized, and many loci with no described association with locomotor or Na(+)/K(+) ATPase function.
Conclusions: We used an unbiased genome-wide screen to find regions of the genome containing elements important for genetic modulation of ATPalpha dysfunction. We have identified many critical regions and narrowed several of these to single genes. These data demonstrate there are many loci capable of modifying ATPalpha dysfunction, which may provide the basis for modifying migraine, locomotor and seizure dysfunction in animals.
Figures
References
-
- Lingrel JB. Na, K-ATPase: isoform structure, function, and expression. J Bioenerg Biomembr. 1992;24:263–270. - PubMed
-
- Lopina OD. Na+, K + −ATPase: structure, mechanism, and regulation. Membr Cell Biol. 2000;13:721–744. - PubMed
-
- Skou JC, Esmann M. The Na, K-ATPase. J Bioenerg Biomembr. 1992;24:249–261. - PubMed
-
- Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, Tiziano FD, Fontaine B, Walley NM, Heavin S, Panagiotakaki E, European Alternating Hemiplegia of Childhood (AHC) Genetics Consortium; Biobanca e Registro Clinico per l'Emiplegia Alternante (I.B.AHC) Consortium; European Network for Research on Alternating Hemiplegia (ENRAH) for Small and Medium-sized Enterpriese (SMEs) Consortium, Fiori S, Abiusi E, Di Pietro L, Sweney MT, Newcomb TM, Viollet L, Huff C, Jorde LB, Reyna SP, Murphy KJ, Shianna KV, Gumbs CE, Little L, Silver K, Ptáček LJ, Haan J: De novo mutations in ATP1A3 cause alternating hemiplegia of childhood.Nat Genetᅟ, 44:1030–1034. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

