TBCRC 008: early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer
- PMID: 25476537
- PMCID: PMC4352313
- DOI: 10.2967/jnumed.114.144741
TBCRC 008: early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer
Abstract
Epigenetic modifiers, including the histone deacetylase inhibitor vorinostat, may sensitize tumors to chemotherapy and enhance outcomes. We conducted a multicenter randomized phase II neoadjuvant trial of carboplatin and nanoparticle albumin-bound paclitaxel (CP) with vorinostat or placebo in women with stage II/III, human epidermal growth factor receptor 2 (HER2)-negative breast cancer, in which we also examined whether change in maximum standardized uptake values corrected for lean body mass (SUL(max)) on (18)F-FDG PET predicted pathologic complete response (pCR) in breast and axillary lymph nodes.
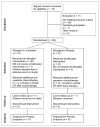
Methods: Participants were randomly assigned to 12 wk of preoperative carboplatin (area under the curve of 2, weekly) and nab-paclitaxel (100 mg/m(2) weekly) with vorinostat (400 mg orally daily, days 1-3 of every 7-d period) or placebo. All patients underwent (18)F-FDG PET and research biopsy at baseline and on cycle 1 day 15. The primary endpoint was the pCR rate. Secondary objectives included correlation of change in tumor SUL(max) on (18)F-FDG PET by cycle 1 day 15 with pCR and correlation of baseline and change in Ki-67 with pCR.
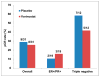
Results: In an intent-to-treat analysis (n = 62), overall pCR was 27.4% (vorinostat, 25.8%; placebo, 29.0%). In a pooled analysis (n = 59), we observed a significant difference in median change in SUL(max) 15 d after initiating preoperative therapy between those achieving pCR versus not (percentage reduction, 63.0% vs. 32.9%; P = 0.003). Patients with 50% or greater reduction in SUL(max) were more likely to achieve pCR, which remained statistically significant in multivariable analysis including estrogen receptor status (odds ratio, 5.1; 95% confidence interval, 1.3-22.7; P = 0.023). Differences in baseline and change in Ki-67 were not significantly different between those achieving pCR versus not.
Conclusion: Preoperative CP with vorinostat or placebo is associated with similar pCR rates. Early change in SUL(max) on (18)F-FDG PET 15 d after the initiation of preoperative therapy has potential in predicting pCR in patients with HER2-negative breast cancer. Future studies will further test (18)F-FDG PET as a potential treatment-selection biomarker.
Keywords: 18F-FDG PET; biomarker; breast cancer; neoadjuvant; vorinostat.
© 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Figures

Comment in
-
Measuring tumor metabolism by 18F-FDG PET predicts outcome in a multicenter study: a step off in the right direction.J Nucl Med. 2015 Jan;56(1):1-2. doi: 10.2967/jnumed.114.151175. J Nucl Med. 2015. PMID: 25537988 No abstract available.
References
-
- Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001;61:8492–8497. - PubMed
-
- Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. - PubMed
-
- Ramalingam SS, Parise RA, Ramanathan RK, et al. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res. 2007;13:3605–3610. - PubMed
-
- Wolff AC, Berry D, Carey LA, et al. Research issues affecting preoperative systemic therapy for operable breast cancer. J Clin Oncol. 2008;26:806–813. - PubMed
-
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
