Human mesenchymal stem cells possess different biological characteristics but do not change their therapeutic potential when cultured in serum free medium
- PMID: 25476802
- PMCID: PMC4445567
- DOI: 10.1186/scrt522
Human mesenchymal stem cells possess different biological characteristics but do not change their therapeutic potential when cultured in serum free medium
Abstract
Introduction: Mesenchymal stem cells (MSCs) are widely investigated in clinical researches to treat various diseases. Classic culture medium for MSCs, even for clinical use, contains fetal bovine serum. The serum-containing medium (SCM) seems a major obstacle for MSCs-related therapies due to the risk of contamination of infectious pathogens. Some studies showed that MSCs could be expanded in serum free medium (SFM); however, whether SFM would change the biological characteristics and safety issues of MSCs has not been well answered.
Methods: Human umbilical cord mesenchymal stem cells (hUC-MSCs) were cultured in a chemical defined serum free medium. Growth, multipotency, surface antigen expression, telomerase, immunosuppressive ability, gene expression profile and genomic stability of hUC-MSCs cultured in SFM and SCM were analyzed and compared side by side.
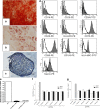
Results: hUC-MSCs propagated more slowly and senesce ultimately in SFM. SFM-expanded hUC-MSCs were different from SCM-expanded hUC-MSCs in growth rate, telomerase, gene expression profile. However, SFM-expanded hUC-MSCs maintained multipotency and the profile of surface antigen which were used to define human MSCs. Both SFM- and SCM-expanded hUC-MSCs gained copy number variation (CNV) in long-term in vitro culture.
Conclusion: hUC-MCSs could be expanded in SFM safely to obtain enough cells for clinical application, meeting the basic criteria for human mesenchymal stem cells. hUC-MSCs cultured in SFM were distinct from hUC-MSCs cultured in SCM, yet they remained therapeutic potentials for future regenerative medicine.
Figures




Similar articles
-
Evaluation of the impact of customized serum-free culture medium on the production of clinical-grade human umbilical cord mesenchymal stem cells: insights for future clinical applications.Stem Cell Res Ther. 2024 Sep 27;15(1):327. doi: 10.1186/s13287-024-03949-0. Stem Cell Res Ther. 2024. PMID: 39334391 Free PMC article.
-
Native extracellular matrix preserves mesenchymal stem cell "stemness" and differentiation potential under serum-free culture conditions.Stem Cell Res Ther. 2015 Dec 1;6:235. doi: 10.1186/s13287-015-0235-6. Stem Cell Res Ther. 2015. PMID: 26620283 Free PMC article.
-
Isolation, expansion and characterization of bone marrow-derived mesenchymal stromal cells in serum-free conditions.Cell Tissue Res. 2014 Apr;356(1):123-35. doi: 10.1007/s00441-013-1783-7. Epub 2014 Jan 22. Cell Tissue Res. 2014. PMID: 24448665
-
Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy.Expert Opin Biol Ther. 2015;15(9):1293-306. doi: 10.1517/14712598.2015.1051528. Epub 2015 Jun 12. Expert Opin Biol Ther. 2015. PMID: 26067213 Review.
-
Human Umbilical Cord Mesenchymal Stem Cells' Cultivation and Treatment of Liver Diseases.Curr Stem Cell Res Ther. 2023;18(3):286-298. doi: 10.2174/1574888X17666220623111406. Curr Stem Cell Res Ther. 2023. PMID: 35747963 Review.
Cited by
-
An efficient protocol to generate placental chorionic plate-derived mesenchymal stem cells with superior proliferative and immunomodulatory properties.Stem Cell Res Ther. 2019 Oct 17;10(1):301. doi: 10.1186/s13287-019-1405-8. Stem Cell Res Ther. 2019. PMID: 31623677 Free PMC article.
-
Type I collagen facilitates safe and reliable expansion of human dental pulp stem cells in xenogeneic serum-free culture.Stem Cell Res Ther. 2020 Jul 14;11(1):267. doi: 10.1186/s13287-020-01776-7. Stem Cell Res Ther. 2020. PMID: 32660544 Free PMC article.
-
Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats.Stem Cell Res Ther. 2021 Jan 19;12(1):69. doi: 10.1186/s13287-020-02130-7. Stem Cell Res Ther. 2021. PMID: 33468246 Free PMC article.
-
Accumulating Transcriptome Drift Precedes Cell Aging in Human Umbilical Cord-Derived Mesenchymal Stromal Cells Serially Cultured to Replicative Senescence.Stem Cells Transl Med. 2019 Sep;8(9):945-958. doi: 10.1002/sctm.18-0246. Epub 2019 Mar 28. Stem Cells Transl Med. 2019. PMID: 30924318 Free PMC article.
-
Serum- and xeno-free culture of human umbilical cord perivascular cells for pediatric heart valve tissue engineering.Stem Cell Res Ther. 2023 Apr 19;14(1):96. doi: 10.1186/s13287-023-03318-3. Stem Cell Res Ther. 2023. PMID: 37076906 Free PMC article.
References
-
- Cancedda R, Mastrogiacomo M, Bianchi G, Derubeis A, Muraglia A, Quarto R. Bone marrow stromal cells and their use in regenerating bone. Novartis Found Symp. 2003;249:133–143. - PubMed
-
- Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. - DOI - PubMed
-
- Karlsson H, Samarasinghe S, Ball LM, Sundberg B, Lankester AC, Dazzi F, Uzunel M, Rao K, Veys P, Le Blanc K, Ringdén O, Amrolia PJ. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T-cell responses. Blood. 2008;112:532–541. doi: 10.1182/blood-2007-10-119370. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

