The habenulo-interpeduncular pathway in nicotine aversion and withdrawal
- PMID: 25476971
- PMCID: PMC4452453
- DOI: 10.1016/j.neuropharm.2014.11.019
The habenulo-interpeduncular pathway in nicotine aversion and withdrawal
Abstract
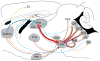
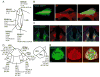
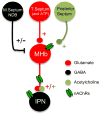
Progress has been made over the last decade in our understanding of the brain areas and circuits involved in nicotine reward and withdrawal, leading to models of addiction that assign different addictive behaviors to distinct, yet overlapping, neural circuits (Koob and Volkow, 2010; Lobo and Nestler, 2011; Tuesta et al., 2011; Volkow et al., 2011). Recently the habenulo-interpeduncular (Hb-IPN) midbrain pathway has re-emerged as a new critical crossroad that influences the brain response to nicotine. This brain area is particularly enriched in nicotinic acetylcholine receptor (nAChR) subunits α5, α3 and β4 encoded by the CHRNA5-A3-B4 gene cluster, which has been associated with vulnerability to tobacco dependence in human genetics studies. This finding, together with studies in mice involving deletion and replacement of nAChR subunits, and investigations of the circuitry, cell types and electrophysiological properties, have begun to identify the molecular mechanisms that take place in the MHb-IPN which underlie critical aspects of nicotine dependence. In the current review we describe the anatomical and functional connections of the MHb-IPN system, as well as the contribution of specific nAChRs subtypes in nicotine-mediated behaviors. Finally, we discuss the specific electrophysiological properties of MHb-IPN neuronal populations and how nicotine exposure alters their cellular physiology, highlighting the unique role of the MHb-IPN in the context of nicotine aversion and withdrawal. This article is part of the Special Issue entitled 'The Nicotinic Acetylcholine Receptor: From Molecular Biology to Cognition'.
Keywords: Habenula; Interpeduncular nucleus; Nicotine; Pacemaking; nAChR.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Agmon-Snir H, Carr CE, Rinzel J. The role of dendrites in auditory coincidence detection. Nature. 1998;393:268–272. - PubMed
-
- Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–4066. - PubMed
-
- Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407:130–150. - PubMed
-
- Baisden RH, Hoover DB, Cowie RJ. Retrograde demonstration of hippocampal afferents from the interpeduncular and reuniens nuclei. Neurosci Lett. 1979;13:105–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

