Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle
- PMID: 25477138
- PMCID: PMC4300280
- DOI: 10.1016/j.ceca.2014.11.002
Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle
Abstract
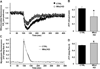
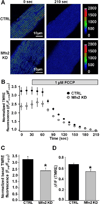
As muscle contraction requires ATP and Ca(2+), skeletal muscle function is highly dependent on communication between two major intracellular organelles: mitochondria and sarcoplasmic reticulum (SR). In adult skeletal muscle, mitochondria located within the I-band of the sarcomere are connected to the SR by small ∼10 nm electron dense tethers that bridge the outer mitochondrial membrane to the region of SR that is ∼130 nm from the site of Ca(2+) release. However, the molecular composition of tethers and their precise impact on mitochondrial Ca(2+) uptake in skeletal muscle is unclear. Mitofusin-2 (Mfn2) is a transmembrane GTPase present in both mitochondria and ER/SR membranes that forms trans-dimers and participates in mitochondrial fusion. Here we evaluated the role Mfn2 plays in mitochondrial morphology, localization, and functional SR-mitochondrial Ca(2+) crosstalk in adult skeletal muscle. Compared to a non-targeting (CTRL) siRNA, in vivo electroporation of 400 nM Mfn2 siRNA (Mfn2 KD) into mouse footpads resulted in a marked acute reduction (67±3%) in Mfn2 protein levels in flexor digitorum brevis (FDB) muscles that occurred without a change in other key Ca(2+) regulatory proteins. Electron microscopy analyses revealed that Mfn2 knockdown resulted in a change in mitochondria morphology and mis-localization of some mitochondria from the I-band to the A-band region of the sarcomere. To assess the role of Mfn2 in SR-mitochondrial crosstalk, we measured mitochondrial Ca(2+) uptake and myoplasmic Ca(2+) transients with rhod-2 and mag-fluo-4, respectively, during repetitive high frequency tetanic stimulation (5×100 Hz tetani, 500 ms/tetani, 0.2 duty cycle) in CTRL and Mfn2 KD fibers. Mitochondrial Ca(2+) uptake during repetitive tetanic stimulation was significantly reduced (40%) in Mfn2 KD FDB fibers, which was accompanied by a parallel elevation in the global electrically evoked myoplasmic Ca(2+) transient. Mfn2 KD also resulted in a reduction of the mitochondrial membrane potential, which contributed to the observed decrease in activity-dependent mitochondrial Ca(2+) uptake. Consistent with this idea, a similar decrease in mitochondrial Ca(2+) uptake was also observed in wild type fibers following a comparable reduction in mitochondrial membrane potential induced by acute exposure to a low concentration (50 nM) of carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP). In addition, both global and mitochondrial Ca(2+) transients during repetitive tetanic stimulation were similarly reduced by both slow (EGTA) and fast (BAPTA) Ca(2+) chelating agents. Together, these results indicate that Mfn2 promotes proper mitochondrial morphology, localization, and membrane potential required for optimal activity-dependent mitochondrial Ca(2+) uptake and buffering of the global myoplasmic Ca(2+) transient in adult skeletal muscle.
Keywords: Calcium signaling; Mitochondrial calcium uptake; Mitochondrial membrane potential; Mitofusin; Skeletal muscle; Tether.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
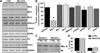
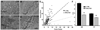



References
-
- Westerblad H, Bruton JD, Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res. 2010;316(18):3093–3099. - PubMed
-
- Heymsfield SB, et al. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282(1):E132–E138. - PubMed
-
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. - PubMed
-
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114(Pt 5):867–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

