Microfluidics-based selection of red-fluorescent proteins with decreased rates of photobleaching
- PMID: 25477249
- PMCID: PMC4323946
- DOI: 10.1039/c4ib00251b
Microfluidics-based selection of red-fluorescent proteins with decreased rates of photobleaching
Abstract
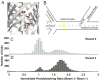
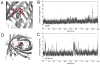
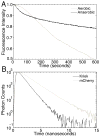
Fluorescent proteins offer exceptional labeling specificity in living cells and organisms. Unfortunately, their photophysical properties remain far from ideal for long-term imaging of low-abundance cellular constituents, in large part because of their poor photostability. Despite widespread engineering efforts, improving the photostability of fluorescent proteins remains challenging due to lack of appropriate high-throughput selection methods. Here, we use molecular dynamics guided mutagenesis in conjunction with a recently developed microfluidic-based platform, which sorts cells based on their fluorescence photostability, to identify red fluorescent proteins with decreased photobleaching from a HeLa cell-based library. The identified mutant, named Kriek, has 2.5- and 4-fold higher photostability than its progenitor, mCherry, under widefield and confocal illumination, respectively. Furthermore, the results provide insight into mechanisms for enhancing photostability and their connections with other photophysical processes, thereby providing direction for ongoing development of fluorescent proteins with improved single-molecule and low-copy imaging capabilities.
Figures
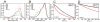
Similar articles
-
Directed evolution of excited state lifetime and brightness in FusionRed using a microfluidic sorter.Integr Biol (Camb). 2018 Sep 17;10(9):516-526. doi: 10.1039/c8ib00103k. Integr Biol (Camb). 2018. PMID: 30094420 Free PMC article.
-
Cysteine Sulfoxidation Increases the Photostability of Red Fluorescent Proteins.ACS Chem Biol. 2016 Oct 21;11(10):2679-2684. doi: 10.1021/acschembio.6b00579. Epub 2016 Sep 12. ACS Chem Biol. 2016. PMID: 27603966 Free PMC article.
-
Spontaneously Blinking Fluorescent Protein for Simple Single Laser Super-Resolution Live Cell Imaging.ACS Chem Biol. 2018 Aug 17;13(8):1938-1943. doi: 10.1021/acschembio.8b00200. Epub 2018 Jul 10. ACS Chem Biol. 2018. PMID: 29963852
-
Structure-guided rational design of red fluorescent proteins: towards designer genetically-encoded fluorophores.Curr Opin Struct Biol. 2017 Aug;45:91-99. doi: 10.1016/j.sbi.2016.12.001. Epub 2016 Dec 27. Curr Opin Struct Biol. 2017. PMID: 28038355 Review.
-
Deciphering Structural Photophysics of Fluorescent Proteins by Kinetic Crystallography.Int J Mol Sci. 2017 Jun 2;18(6):1187. doi: 10.3390/ijms18061187. Int J Mol Sci. 2017. PMID: 28574447 Free PMC article. Review.
Cited by
-
Enhancing fluorescent protein photostability through robot-assisted photobleaching.Integr Biol (Camb). 2018 Jul 16;10(7):419-428. doi: 10.1039/c8ib00063h. Integr Biol (Camb). 2018. PMID: 29897363 Free PMC article.
-
Directed evolution of excited state lifetime and brightness in FusionRed using a microfluidic sorter.Integr Biol (Camb). 2018 Sep 17;10(9):516-526. doi: 10.1039/c8ib00103k. Integr Biol (Camb). 2018. PMID: 30094420 Free PMC article.
-
Rapid directed molecular evolution of fluorescent proteins in mammalian cells.Protein Sci. 2022 Mar;31(3):728-751. doi: 10.1002/pro.4261. Epub 2021 Dec 30. Protein Sci. 2022. PMID: 34913537 Free PMC article.
-
Automating the High-Throughput Screening of Protein-Based Optical Indicators and Actuators.Biochemistry. 2023 Jan 17;62(2):169-177. doi: 10.1021/acs.biochem.2c00357. Epub 2022 Oct 31. Biochemistry. 2023. PMID: 36315460 Free PMC article. Review.
-
Flow-Based Single Cell Deposition for High-Throughput Screening of Protein Libraries.PLoS One. 2015 Nov 4;10(11):e0140730. doi: 10.1371/journal.pone.0140730. eCollection 2015. PLoS One. 2015. PMID: 26536118 Free PMC article.
References
-
- Shu X, Shaner NC, Yarbrough CA, Tsien RY, Remington SJ. Biochemistry. 2006;45:9639–9647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

