The methyltransferases enhancer of zeste homolog (EZH) 1 and EZH2 control hepatocyte homeostasis and regeneration
- PMID: 25477280
- PMCID: PMC4415007
- DOI: 10.1096/fj.14-261537
The methyltransferases enhancer of zeste homolog (EZH) 1 and EZH2 control hepatocyte homeostasis and regeneration
Abstract
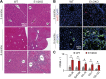
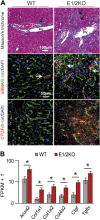
To investigate the role of enhancer of zeste homolog (EZH) 1 and EZH2 in liver homeostasis, mice were generated that carried Ezh1(-/-) and EZH2(fl/fl) alleles and an Alb-Cre transgene. Only the combined loss of EZH1 and EZH2 in mouse hepatocytes caused a depletion of global trimethylation on Lys 27 of histone H3 (H3K27me3) marks and the specific loss of over ∼1900 genes at 3 mo of age. Ezh1(-/-),Ezh2(fl/fl)Alb-Cre mice exhibited progressive liver abnormalities manifested by the development of regenerative nodules and concomitant periportal fibrosis, inflammatory infiltration, and activation of A6-positive hepatic progenitor cells at 8 mo of age. In response to chronic treatment with carbon tetrachloride, all experimental mice, but none of the controls (n = 27 each), showed increased hepatic degeneration associated with liver dysfunction and reduced ability to proliferate. After two-thirds partial hepatectomy, mutant mice (n = 5) displayed increased liver injury and a blunted regenerative response. Genome-wide analyses at 3 mo of age identified 51 genes that had lost H3K27me3 marks, and their expression was significantly increased. These genes were involved in regulation of cell survival, fibrosis, and proliferation. H3K27me3 levels and liver physiology were unaffected in mice lacking either EZH1 globally or EZH2 specifically in hepatocytes. This work demonstrates a critical redundancy of EZH1 and EZH2 in maintaining hepatic homeostasis and regeneration.
Keywords: H3-K27 trimethylation; fibrosis; liver regeneration.
© FASEB.
Figures





References
-
- Ringrose L., Paro R. (2004) Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu. Rev. Genet. 38, 413–443 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

