Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation
- PMID: 25477520
- PMCID: PMC4326810
- DOI: 10.1074/jbc.R114.607044
Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation
Abstract
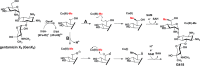
Radical S-adenosylmethionine (SAM) enzymes use the oxidizing power of a 5'-deoxyadenosyl 5'-radical to initiate an amazing array of transformations, usually through the abstraction of a target substrate hydrogen atom. A common reaction of radical SAM (RS) enzymes is the methylation of unactivated carbon or phosphorous atoms found in numerous primary and secondary metabolites, as well as in proteins, sugars, lipids, and RNA. However, neither the chemical mechanisms by which these unactivated atoms obtain methyl groups nor the actual methyl donors are conserved. In fact, RS methylases have been grouped into three classes based on protein architecture, cofactor requirement, and predicted mechanism of catalysis. Class A methylases use two cysteine residues to methylate sp(2)-hybridized carbon centers. Class B methylases require a cobalamin cofactor to methylate both sp(2)-hybridized and sp(3)-hybridized carbon centers as well as phosphinate phosphorous atoms. Class C methylases share significant sequence homology with the RS enzyme, HemN, and may bind two SAM molecules simultaneously to methylate sp(2)-hybridized carbon centers. Lastly, we describe a new class of recently discovered RS methylases. These Class D methylases, unlike Class A, B, and C enzymes, which use SAM as the source of the donated methyl carbon, are proposed to methylate sp(2)-hybridized carbon centers using methylenetetrahydrofolate as the source of the appended methyl carbon.
Keywords: Antibiotics; Cyclopropanation; Iron-Sulfur Protein; Methylation; Methylcobalamin; Methylenetetrahydrofolate; Radical; Ribosomal Ribonucleic Acid (rRNA) (Ribosomal RNA); S-Adenosylmethionine (SAM).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Takusagawa F., Fujioka M., Spies A., Schowen R. L. (1998) S-Adenosylmethionine (AdoMet)-dependent methyltransferases. in Comprehensive Biological Catalysis (Sinnott M. ed), pp. 1–30, Academic Press, New York
-
- Markham G. D. (2010) S-Adenosylmethionine. Encyclopedia of Life Sciences 10.1002/9780470015902.a0000662.pub2 - DOI
-
- Cantoni G. L. (1953) S-Adenosylmethionine: a new intermediate formed enzymatically from l-methionine and adenosine triphosphate. J. Biol. Chem. 204, 403–416 - PubMed
-
- Woodard R. W., Tsai M.-D., Floss H. G., Crooks P. A., Coward J. K. (1980) Sterochemical course of the transmethylation catalyzed by catechol O-methyltransferase. J. Biol. Chem. 255, 9124–9127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources